Exhibit 99.3
LONLON BIOTECH LTD. AND SUBSIDIARIES
CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
March 31, 2021 and 2020
LONLON BIOTECH LTD. AND SUBSIDIARIES
INDEX TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
CONTENTS
F-1
LONLON BIOTECH LTD. AND SUBSIDIARIES
CONDENSED CONSOLIDATED BALANCE SHEETS
| March 31, | December 31, | |||||||
| 2021 | 2020 | |||||||
| (Unaudited) | ||||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash | $ | 188,887 | $ | 18,935 | ||||
| Accounts receivable | 636,746 | 45,271 | ||||||
| Recoverable VAT | 343,755 | 335,150 | ||||||
| Inventory | 105,743 | 125,962 | ||||||
| Prepaid expenses and other current assets | 60,949 | 21,017 | ||||||
| Total Current Assets | 1,336,080 | 546,335 | ||||||
| NON-CURRENT ASSETS: | ||||||||
| Security deposit | 49,843 | 50,012 | ||||||
| Operating lease right-of-use assets, net | 266,406 | 320,123 | ||||||
| Property and equipment, net | 2,268,076 | 2,567,522 | ||||||
| Total Non-current Assets | 2,584,325 | 2,937,657 | ||||||
| Total Assets | $ | 3,920,405 | $ | 3,483,992 | ||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Notes payable | $ | 1,373,480 | $ | 918,752 | ||||
| Notes payable - related party | 244,174 | 245,000 | ||||||
| Accounts payable | 564,192 | 310,330 | ||||||
| Salary payable | 102,329 | 105,810 | ||||||
| Accrued leasehold improvements liabilities | 261,106 | 315,583 | ||||||
| Accrued liabilities and other payables | 62,938 | 37,432 | ||||||
| Deferred revenue | 167,201 | 88,508 | ||||||
| Deferred grant income | 183,496 | 260,679 | ||||||
| Operating lease obligation | 151,167 | 155,470 | ||||||
| Total Current Liabilities | 3,110,083 | 2,437,564 | ||||||
| NON-CURRENT LIABILITIES: | ||||||||
| Deferred grant income - noncurrent portion | 304,617 | 351,677 | ||||||
| Operating lease obligation - noncurrent portion | 52,377 | 105,566 | ||||||
| Total Non-current Liabilities | 356,994 | 457,243 | ||||||
| Total Liabilities | 3,467,077 | 2,894,807 | ||||||
| Commitments and Contingencies | ||||||||
| SHAREHOLDERS’ EQUITY: | ||||||||
| Ordinary shares, $1.00 par value; 50,000 shares authorized; 10,001 shares issued and outstanding at March 31, 2021 and December 31, 2020 * | 10,001 | 10,001 | ||||||
| Additional paid-in capital | 8,946,197 | 8,946,197 | ||||||
| Accumulated deficit | (8,515,294 | ) | (8,380,014 | ) | ||||
| Accumulated other comprehensive income | 12,424 | 13,001 | ||||||
| Total shareholders’ equity | 453,328 | 589,185 | ||||||
| Total Liabilities and Shareholders’ Equity | $ | 3,920,405 | $ | 3,483,992 | ||||
| * | The shares amounts are presented on a retroactive basis. |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-2
LONLON BIOTECH LTD. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(Unaudited)
| For the Three Months Ended | ||||||||
| March 31, | ||||||||
| 2021 | 2020 | |||||||
| REVENUES | ||||||||
| General laboratory testing | $ | 945,648 | $ | 42,887 | ||||
| Immunology and hematology testing | 301,857 | - | ||||||
| Total Revenues | 1,247,505 | 42,887 | ||||||
| COST OF REVENUES | ||||||||
| General laboratory testing | 347,911 | 14,117 | ||||||
| Immunology and hematology testing | 89,498 | - | ||||||
| Total Cost of Revenues | 437,409 | 14,117 | ||||||
| GROSS PROFIT | ||||||||
| General laboratory testing | 597,737 | 28,770 | ||||||
| Immunology and hematology testing | 212,359 | - | ||||||
| Total Gross Profit | 810,096 | 28,770 | ||||||
| OPERATING EXPENSES: | ||||||||
| Research and development expenses | 565,331 | 410,893 | ||||||
| General and administrative expenses | 406,188 | 344,252 | ||||||
| Selling and marketing expenses | 52,707 | 8,584 | ||||||
| Grant income | (123,467 | ) | (526,703 | ) | ||||
| Total Operating Expenses | 900,759 | 237,026 | ||||||
| LOSS FROM OPERATIONS | (90,663 | ) | (208,256 | ) | ||||
| OTHER (EXPENSE) INCOME | ||||||||
| Interest expense | (13,647 | ) | - | |||||
| Interest expense - related party | (2,683 | ) | (1,860 | ) | ||||
| Other income | 226 | 1,444 | ||||||
| Total Other Expense, net | (16,104 | ) | (416 | ) | ||||
| LOSS BEFORE INCOME TAXES | (106,767 | ) | (208,672 | ) | ||||
| INCOME TAXES | 28,513 | - | ||||||
| NET LOSS | $ | (135,280 | ) | $ | (208,672 | ) | ||
| COMPREHENSIVE LOSS: | ||||||||
| NET LOSS | $ | (135,280 | ) | $ | (208,672 | ) | ||
| OTHER COMPREHENSIVE LOSS | ||||||||
| Unrealized foreign currency translation loss | (577 | ) | (47,085 | ) | ||||
| COMPREHENSIVE LOSS | $ | (135,857 | ) | $ | (255,757 | ) | ||
| NTE LOSS PER ORDINARY SHARE: | ||||||||
| Basis and diluted * | $ | (13.53) | $ | (20.87) | ||||
| WEIGHTED AVERAGE ORDINARY SHARES OUTSTANDING: | ||||||||
| Basis and diluted * | 10,001 | 10,001 | ||||||
| * | The shares and per share amounts are presented on a retroactive basis. |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-3
LONLON BIOTECH LTD. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN SHAEHOLDERS’ EQUITY
FOR THE THREE MONTHS ENDED MARCH 31, 2021
(Unaudited)
| Accumulated | ||||||||||||||||||||||||
| Ordinary Shares * | Additional | Other | Total | |||||||||||||||||||||
| Number of | Paid-in | Accumulated | Comprehensive | Shareholders’ | ||||||||||||||||||||
| Shares | Amount | Capital | Deficit | Income | Equity | |||||||||||||||||||
| Balance as of December 31, 2020 | 10,001 | $ | 10,001 | $ | 8,946,197 | $ | (8,380,014 | ) | $ | 13,001 | $ | 589,185 | ||||||||||||
| Foreign currency translation adjustment | - | - | - | - | (577 | ) | (577 | ) | ||||||||||||||||
| Net loss for the three months ended March 31, 2021 | - | - | - | (135,280 | ) | - | (135,280 | ) | ||||||||||||||||
| Balance as of March 31, 2021 (unaudited) | 10,001 | $ | 10,001 | $ | 8,946,197 | $ | (8,515,294 | ) | $ | 12,424 | $ | 453,328 | ||||||||||||
| * | The shares amounts are presented on a retroactive basis. |
LONLON BIOTECH LTD. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN SHAEHOLDERS’ EQUITY
FOR THE THREE MONTHS ENDED MARCH 31, 2020
(Unaudited)
| Accumulated | ||||||||||||||||||||||||
| Ordinary Shares* | Other | Total | ||||||||||||||||||||||
| Number of | Paid-in | Accumulated | Comprehensive | Shareholders’ | ||||||||||||||||||||
| Shares | Amount | Capital | Deficit | Loss | Equity | |||||||||||||||||||
| Balance as of December 31, 2019 | 10,001 | $ | 10,001 | $ | 8,946,197 | $ | (5,937,651 | ) | $ | (45,806 | ) | $ | 2,972,741 | |||||||||||
| Foreign currency translation adjustment | - | - | - | - | (47,085 | ) | (47,085 | ) | ||||||||||||||||
| Net loss for the three months ended March 31, 2020 | - | - | - | (208,672 | ) | - | (208,672 | ) | ||||||||||||||||
| Balance as of March 31, 2020 (unaudited) | 10,001 | $ | 10,001 | $ | 8,946,197 | $ | (6,146,323 | ) | $ | (92,891 | ) | $ | 2,716,984 | |||||||||||
| * | The shares amounts are presented on a retroactive basis. |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-4
LONLON BIOTECH LTD. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE TRHEE MONTHS ENDED MARCH 31, 2021 AND 2020
(Unaudited)
| For the Three Months Ended | ||||||||
| March 31, | ||||||||
| 2021 | 2020 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | (135,280 | ) | $ | (208,672 | ) | ||
| Adjustments to reconcile net loss to net cash (used in) provided by operating activities: | ||||||||
| Depreciation | 297,458 | 252,099 | ||||||
| Amortization of right-of-use assets | 53,193 | 44,912 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivable | (597,869 | ) | (1,232 | ) | ||||
| Recoverable VAT | (9,838 | ) | (14,661 | ) | ||||
| Inventory | 20,002 | (9,015 | ) | |||||
| Prepaid expenses and other current assets | (40,425 | ) | 32,917 | |||||
| Security deposit | - | 1,432 | ||||||
| Accounts payable | 257,598 | 35,052 | ||||||
| Salary payable | (3,157 | ) | (10,788 | ) | ||||
| Accrued liabilities and other payables | 25,902 | (22,052 | ) | |||||
| Interest payable - related party | - | 1,860 | ||||||
| Deferred revenue | 79,824 | 135 | ||||||
| Deferred grant income | (123,467 | ) | (42,622 | ) | ||||
| Operating lease obligation | (57,208 | ) | (45,556 | ) | ||||
| NET CASH (USED IN) PROVIDED BY OPERATING ACTIVITIES | (233,267 | ) | 13,809 | |||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Purchase of property and equipment | (57,581 | ) | (18,665 | ) | ||||
| NET CASH USED IN INVESTING ACTIVITIES | (57,581 | ) | (18,665 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Proceeds from borrowings | 462,656 | - | ||||||
| Proceeds from related party’s borrowings | - | 214,829 | ||||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES | 462,656 | 214,829 | ||||||
| EFFECT OF EXCHANGE RATE ON CASH | (1,856 | ) | (5,744 | ) | ||||
| NET INCREASE IN CASH | 169,952 | 204,229 | ||||||
| CASH - beginning of period | 18,935 | 164,994 | ||||||
| CASH - end of period | $ | 188,887 | $ | 369,223 | ||||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | ||||||||
| Cash paid for: | ||||||||
| Interest | $ | 10,292 | $ | - | ||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-5
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 1 – ORGANIZATION AND NATURE OF OPERATIONS
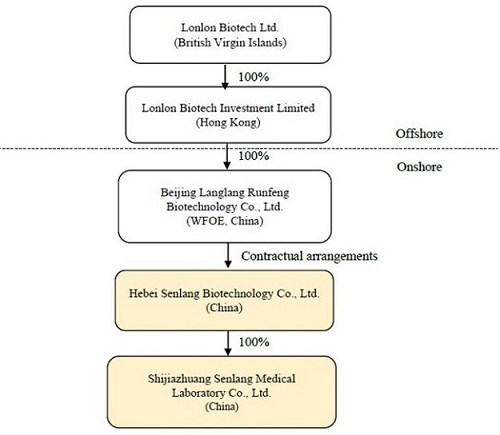
Lonlon Biotech Ltd. (“Senlang” or the “Company”) is a holding company incorporated in the British Virgin Islands (“BVI”) on October 15, 2020. The Company is mainly engaged in the business of research and development in relation to CAR-T cell therapy, immune cell therapy and related drug development in the People’s Republic of China (“PRC” or “China”), through a variable interest entity (“VIE” as defined in Note 4), Hebei Senlang Biotechnology Co., Ltd. (“SenlangBio”), which was established on January 29, 2016, and subsidiary of the VIE. SenlangBio also provides immunology and hematology testing services, mainly related to cell therapy, including tumor biomarkers and immunophenotyping. On February 9, 2018, SenlangBio formed a wholly owned subsidiary, Shijiazhuang Senlang Medical Laboratory Co., Ltd. (“SenlangBio Clinical Laboratory”), in China, which focuses on general laboratory testing for patients and other customers, including genomics, proteomics, routine blood/urine testing, COVID-19 PCR/antibody testing etc.
On November 2, 2020, Senlang established a wholly owned subsidiary in Hong Kong, Lonlon Biotech Investment Limited (“Senlang HK”), which is a holding company. On November 20, 2020, Senlang HK established a Wholly Foreign-Owned Enterprise in China, Beijing Langlang Runfeng Biotechnology Co., Ltd. (“Senlang BJ” or “WFOE”).
On April 26, 2021, Senlang BJ entered into a series of contractual arrangements, or VIE agreements with SenlangBio and 13 equity holders of SenlangBio, through which the Company obtained control and became the primary beneficiary of SenlangBio, hereinafter referred to as the Reorganization. As a result, SenlangBio became the Company’s VIE.
On April 26, 2021, the Company completed its reorganization of the entities under the common control of 13 majority shareholders through their 100% controlled entities incorporated in the British Virgin Islands, and indirectly owned a majority of the equity interests of the Company, its subsidiaries, its VIE and the VIE’s subsidiary prior to and after the Reorganization. The Company was established as a holding company of Senlang BJ. Senlang BJ is the primary beneficiary of SenlangBio, and all of these entities are under common control of the Company’s ultimate controlling shareholders before and after the Reorganization, which results in the consolidation of the Company and has been accounted for as a reorganization of entities under common control at carrying value and for accounting purpose, the reorganization was accounted for as a recapitalization. The consolidated financial statements are prepared on the basis as if the Reorganization became effective as of the beginning of the first period presented in the accompanying consolidated financial statements of the Company.
The following chart illustrates the Company’s corporate structure, including its subsidiaries, consolidated variable interest entity and VIE’s subsidiary as of the issuance date of this report:

F-6
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 1 – ORGANIZATION AND NATURE OF OPERATIONS (continued)
VIE Agreements with SenlangBio
Upon the completion of the reorganization, the Company, through the WFOE, entered into the following contractual arrangements with the VIE and the VIE’s 13 shareholders that enabled the Company to (1) have power to direct the activities that most significantly affects the economic performance of the VIE, and (2) receive the economic benefits of the VIE that could be significant to the VIE. Accordingly, the WFOE was considered the primary beneficiary of the VIE and had consolidated the VIE and the VIE’s subsidiary’ financial results of operations, assets and liabilities in the Company’s consolidated financial statements.
Contracts that give the Company effective control of the VIE
Equity Pledge Agreement
Under the Equity Pledge Agreement between Senlang BJ, SenlangBio and SenlangBio’s shareholders, SenlangBio’s shareholders agree to pledge all of their equity interests in SenlangBio to Senlang BJ to guarantee the performance of SenlangBio and SenlangBio’s shareholders’ obligations under the Exclusive Technical Consultation and Service Agreement, the Exclusive Purchase Option Agreement, the Shareholder’s Rights Proxy Agreement, and the Spousal Consent (“Transaction Agreements”). Under the terms of the Equity Pledge Agreement, in the event that SenlangBio or SenlangBio’s shareholders breach their respective contractual obligations under the Transaction Agreements or the Equity Pledge Agreement, Senlang BJ, as pledgee, is entitled to directly exercise the pledge right and to notify SenlangBio’s shareholders to immediately repay or pay the loans or other payables under the Transaction Agreements. SenlangBio’s shareholders further agreed not to dispose of the pledged equity interests without prior written consent from Senlang BJ.
The pledge is effective on the date when the registration of the equity pledge is completed with the competent administration for industry and commerce, and the term of validity of the pledge is the same as the longest term of validity in the Transaction Agreements.
The purposes of the Equity Pledge Agreement are to (1) guarantee the performance of SenlangBio and SenlangBio’s shareholders’ obligations under the Transaction Agreements, (2) make sure SenlangBio’s shareholders do not transfer or assign the pledged equity interests, or create or allow any encumbrance that would prejudice interests of Senlang BJ without prior written consent from Senlang BJ, and (3) provide Senlang BJ control over SenlangBio.
In the event SenlangBio or SenlangBio’s shareholders breaches their contractual obligations under the Transaction Agreements or Equity Pledge Agreement, Senlang BJ will be entitled to (1) be compensated on a preferential basis with the proceeds from the conversion, auction or sale of the pledged equity, and (2) notify SenlangBio’s shareholders to immediately repay the loans or other payables under the Transaction Agreements.
Exclusive Purchase Option Agreement
Under the Exclusive Purchase Option Agreement, SenlangBio’s shareholders irrevocably grants Senlang BJ (or its designee) an exclusive right to purchase the equity held by SenlangBio’s shareholders in the SenlangBio in whole or in part at any time during the term of the Agreement; SenlangBio also irrevocably grants Senlang BJ (or its designee) an exclusive right to purchase the assets owned by SenlangBio in whole or in part at any time during the term of the Agreement.
With respect to consideration for equity purchase, Senlang BJ has the right to purchase all or part of the equity held by SenlangBio’s shareholders in SenlangBio at the lowest price permitted by the PRC laws. With respect to the price for asset purchase, Senlang BJ has the right to purchase SenlangBio’s assets at a price equivalent of the net book value of the purchased assets; provided that if the minimum price permitted by the PRC law is higher than the net book value, the minimum price permitted by the PRC laws will prevail.
Under the Exclusive Purchase Option Agreement, Senlang BJ may purchase all or part of the equity held by SenlangBio’s shareholders in SenlangBio and all or part of the assets owned by SenlangBio at any time to the extent permitted by PRC laws. The Exclusive Purchase Option Agreement, together with the Equity Pledge Agreement, Exclusive Technical Consultation and Service Agreement, and the Proxy Agreement, enable Senlang BJ to exercise effective control over SenlangBio. The Exclusive Purchase Option Agreement will become effective upon the affixation of signatures or corporate seals by the Senlang BJ, SenlangBio and SenlangBio’s shareholders, and will remain in effect, unless terminated by Senlang BJ by giving a thirty (30) day advance written notice.
F-7
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 1 – ORGANIZATION AND NATURE OF OPERATIONS (continued)
Contracts that give the Company effective control of the VIE
Shareholder’s Rights Proxy Agreement
Under the Shareholder’s Rights Proxy Agreement, SenlangBio’s shareholders authorize any entity or individual designated by Senlang BJ to act on their behalf as their exclusive agent to exercise all shareholder’s rights under the PRC laws and the Articles of Association, including but not limited to: (a) to convene, attend and vote on all the matters during shareholders’ meetings; (b) to transfer, pledge or dispose of, or create encumbrance on the equity; (c) to receive dividends; (d) to participate in judicial proceedings or execute legal documents in relation to shareholders’ rights; (e) to appoint legal representatives, directors and officers of SenlangBio; and (f) to enter into contracts and exercise the Exclusive Purchase Option Agreement.
The Shareholder’s Rights Proxy Agreement shall remain effective until the earlier of: (a) the date on which SenlangBio’s shareholders are no longer the nominee or actual shareholders of Senlang BJ; (b) the date on which Senlang BJ requests the proxy to be terminated in writing; or (c) the date on which the assets and licenses of SenlangBio have been fully transferred to Senlang BJ.
Contracts that enable the Company to receive substantially all of the economic benefits from the VIE
Exclusive Technical Consultation and Service Agreement
Pursuant to the Exclusive Technical Consultation and Service Agreement between Senlang BJ and SenlangBio, Senlang BJ provides SenlangBio with technical consultation and services, including conducting market research, assisting with developing management and sales plans, implementing relevant technology application, and providing other consultation services for computer network, finance, business, legal affairs, operation, human resources, and other aspects of SenlangBio. Additionally, Senlang BJ agrees to grant SenlangBio its trademarks, software copyrights, management systems, management methods and other intellectual property in relation to its services on a chargeable and revocable basis, but such grant shall not result in the transfer of any intellectual property or create any restriction on Senlang BJ’s full ownership.
For services rendered to SenlangBio under this agreement, Senlang BJ is entitled to collect a service fee calculated based on the complexity of services rendered, time required by Senlang BJ, and the exact contents and commercial value of services rendered. During the term of this agreement, Senlang BJ shall enjoy all the economic benefits derived from SenlangBio’s operation, and in the event of serious difficulties in SenlangBio’s operations, Senlang BJ may provide SenlangBio with financial support, and Senlang BJ has the right to request SenlangBio to cease operation. The Exclusive Technical Consultation and Service Agreement shall remain in effect for ten (10) years and shall be automatically renewed unless it is terminated earlier by Senlang BJ.
Based on the foregoing VIE Agreements, Senlang BJ has effective control of SenlangBio which enables Senlang BJ to receive all of the expected residual returns and absorb the expected losses of the VIE and its subsidiary. Management therefore concludes that the Company, through the above contractual arrangements, has the power to direct the activities that most significantly impact the VIE’s economic performance, bears the risks of and enjoys the rewards normally associated with ownership of the VIE, and therefore the Company is the ultimate primary beneficiary of the VIE. Consequently, the Company consolidates the accounts of SenlangBio and its subsidiary for the periods presented herein, in accordance with Accounting Standards Codification (“ASC”) 810-10, Consolidation. The accompanying consolidated financial statements reflect the activities of Senlang and each of the following entities:
| Name | Background | Ownership | ||
| Subsidiaries: | ||||
| Senlang HK | A Hong Kong company | 100% owned by Senlang | ||
| Incorporated on November 2, 2020 | ||||
| Senlang BJ | A PRC limited liability company and a wholly foreign owned enterprise | 100% owned by Senlang HK | ||
| Incorporated on November 20, 2020 | ||||
| VIE: | ||||
| SenlangBio | A PRC limited liability company | VIE | ||
|
Incorporated on January 29, 2016 Immunology and hematology testing service provider |
||||
| VIE’s subsidiary: | ||||
| SenlangBio | A PRC limited liability company | 100% owned by SenlangBio | ||
| Clinical Laboratory | Incorporated on February 9, 2018 | |||
| General laboratory testing service provider |
F-8
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 2 – BASIS OF PRESENTATION AND GOING CONCERN AND LIQUIDITY
Basis of Presentation
These interim condensed consolidated financial statements of the Company and its subsidiary are unaudited. In the opinion of management, all adjustments (consisting of normal recurring accruals) and disclosures necessary for a fair presentation of these interim condensed consolidated financial statements have been included. The results reported in the condensed consolidated financial statements for any interim periods are not necessarily indicative of the results that may be reported for the entire year. The accompanying condensed consolidated financial statements have been prepared in accordance with the rules and regulations of the Securities and Exchange Commission and do not include all information and footnotes necessary for a complete presentation of financial statements in conformity with accounting principles generally accepted in the United States (“U.S. GAAP”).
The Company’s condensed consolidated financial statements include the accounts of the Company and its subsidiaries, VIE and subsidiary of VIE over which the Company exercises control and, when applicable, entity for which the Company has a controlling financial interest or is the primary beneficiary. All significant intercompany accounts and transactions have been eliminated in consolidation.
Certain information and footnote disclosures normally included in the annual consolidated financial statements prepared in accordance with U.S. GAAP have been condensed or omitted. These condensed consolidated financial statements should be read in conjunction with the Company’s audited consolidated financial statements and notes thereto included in elsewhere in this 8K/A.
Going Concern and Liquidity
The Company currently has limited operations. Currently, the Company’s operations are focused on utilizing cell and gene engineering technologies to generate innovative and transformative cellular immunotherapies for solid and hematologic cancers. The Company provides general laboratory testing and immunology and hematology testing services for patients and other customers in China. These consolidated financial statements have been prepared assuming that the Company will continue as a going concern, which contemplates, among other things, the realization of assets and the satisfaction of liabilities in the normal course of business.
As reflected in the accompanying condensed consolidated financial statements, the Company had a working capital deficit of $1,774,003 at March 31, 2021, and has incurred net loss and negative cash flow from operating activities of $135,280 and $233,267, respectively, for the three months ended March 31, 2021. The Company has a limited operating history and its continued growth is dependent upon the continuation of providing general laboratory testing and immunology and hematology testing services, and obtaining additional financing to fund future obligations and pay liabilities arising from normal business operations. In addition, the current cash balance cannot be projected to cover the operating expenses for the next twelve months from the release date of this report. These matters raise substantial doubt about the Company’s ability to continue as a going concern. The ability of the Company to continue as a going concern is dependent on the Company’s ability to raise additional capital, implement its business plan, and generate significant revenues. There are no assurances that the Company will be successful in its efforts to generate significant revenues, maintain sufficient cash balance or report profitable operations or to continue as a going concern. The Company plans on raising capital through bank and other borrowings to implement its business plan. However, there is no assurance these plans will be realized and that any additional financings will be available to the Company on satisfactory terms and conditions, if any. These condensed consolidated financial statements have been prepared assuming that the Company will continue as a going concern, which contemplates, among other things, the realization of assets and the satisfaction of liabilities in the normal course of business.
The occurrence of an uncontrollable event such as the COVID-19 pandemic could negatively impact the Company’s operations even though the pandemic did not significantly impact the Company’s operation in the first quarter of 2021. However, given the dynamic nature of these circumstances, the uncertainty around the potential resurgence of the COVID-19 cases in China, and the instability of local policies and restrictions, the COVID-19 impact over the Company’s business in the rest of year 2021 cannot be reasonably estimated at this time. If COVID-19 cases resurged in the area the Company conducted its business and local government implemented new restrictions in the effort to contain the spread, it is expected the Company’s business will be negatively impacted.
The accompanying condensed consolidated financial statements do not include any adjustments related to the recoverability or classification of asset-carrying amounts or the amounts and classification of liabilities that may result should the Company be unable to continue as a going concern.
F-9
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Use of Estimates and Assumptions
The preparation of the condensed consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from these estimates. Significant estimates during the three months ended March 31, 2021 and 2020 include the useful lives of property and equipment, assumptions used in assessing impairment of long-term assets, and valuation of deferred tax assets and the associated valuation allowances.
Fair Value of Financial Instruments and Fair Value Measurements
The Company adopted the guidance of Accounting Standards Codification (“ASC”) 820 for fair value measurements which clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
| ● | Level 1-Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date. |
| ● | Level 2-Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other than quoted prices that are observable, and inputs derived from or corroborated by observable market data. |
| ● | Level 3-Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information. |
The carrying values of current assets and current liabilities in the Company’s condensed consolidated balance sheets approximated their fair values as of March 31, 2021 and December 31, 2020 due to their short-term nature.
ASC 825-10 “Financial Instruments”, allows entities to voluntarily choose to measure certain financial assets and liabilities at fair value (fair value option). The fair value option may be elected on an instrument-by-instrument basis and is irrevocable, unless a new election date occurs. If the fair value option is elected for an instrument, unrealized gains and losses for that instrument should be reported in earnings at each subsequent reporting date. The Company did not elect to apply the fair value option to any outstanding instruments.
Cash
Cash consists of cash on hand and cash in the bank. The Company maintains cash with financial institutions in the PRC.
Credit Risk and Uncertainties
The Company’s cash is maintained with state-owned banks within the PRC. Balances at state-owned banks within the PRC are covered by insurance up to RMB 500,000 (approximately $76,000) per bank. Any balance over RMB 500,000 per bank in PRC will not be covered. At March 31, 2021, cash balances held by state-owned banks within the PRC are RMB 1,205,456 (approximately $184,000), of which, RMB 172,460 (approximately $26,000) was not covered by such limited insurance. The Company has not experienced any losses in such accounts and believes it is not exposed to any risks on its cash in bank accounts.
The Company’s operations are carried out in PRC. Accordingly, the Company’s business, financial condition and results of operations may be influenced by the political, economic and legal environment in the PRC, and by the general state of the PRC’s economy. The Company’s operations in PRC are subject to specific considerations and significant risks not typically associated with companies in North America. The Company’s results may be adversely affected by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
Financial instruments which potentially subject the Company to concentrations of credit risk consist principally of trade accounts receivable. A portion of the Company’s sales are credit sales which is to the customer whose ability to pay is dependent upon the industry economics prevailing in Hebei province, China; however, concentrations of credit risk with respect to trade accounts receivable is limited due to short term payment terms. The Company also performs ongoing credit evaluations of its customers to help further reduce credit risk.
F-10
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable are presented net of an allowance for doubtful accounts. The Company maintains allowances for doubtful accounts for estimated losses. The Company reviews the accounts receivable on a periodic basis and makes general and specific allowances when there is doubt as to the collectability of individual balances. In evaluating the collectability of individual receivable balances, the Company considers many factors, including the age of the balance, a customer’s payment history, its current credit-worthiness and current economic trends. Accounts are written off after exhaustive efforts at collection.
Management believes that the accounts receivable are fully collectable. Therefore, no allowance for doubtful accounts is deemed to be required on its accounts receivable at March 31, 2021 and December 31, 2020. The Company historically has not experienced significant uncollectible accounts receivable.
Inventory
Inventory consists of raw materials. Inventory is stated at the lower of cost or net realizable value. Cost is determined using the first-in, first-out (FIFO) method. A reserve is established when management determines that certain inventory may not be saleable. If inventory costs exceed expected net realizable value due to obsolescence or quantities in excess of expected demand, the Company will record reserves for the difference between the cost and the estimated net realizable value. The Company did not record any inventory reserve at March 31, 2021 and December 31, 2020.
Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets primarily consist of prepaid utilities and prepaid service fees, which are recognized as expenses over the related service periods. As of March 31, 2021 and December 31, 2020, prepaid expenses and other current assets amounted to $60,949 and $21,017, respectively.
Property and Equipment
Property and equipment are carried at cost and are depreciated on a straight-line basis over the estimated useful lives of the assets. The estimated useful lives are as follows:
| Useful Life | Estimated Residual Value Rate | |||
| Laboratory equipment | 3 - 5 Years | 5% | ||
| Office equipment and furniture | 3 - 5 Years | 5% | ||
| Vehicles | 4 - 5 Years | 5% | ||
| Leasehold improvements | Shorter of the remaining lease terms or estimated useful life | 0% | ||
| Software | 1 - 2 Years | 0% |
The cost of repairs and maintenance is expensed as incurred; major replacements and improvements are capitalized. When assets are retired or disposed of, the cost and accumulated depreciation are removed from the accounts, and any resulting gains or losses are included in income or loss in the period of disposition. The Company examines impairment of fixed assets when events or changes in circumstances reflect the fact that their recorded value may not be recoverable.
Impairment of Long-lived Assets
In accordance with ASC Topic 360, the Company reviews long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable, or at least annually. The Company recognizes an impairment loss when the sum of expected undiscounted future cash flows is less than the carrying amount of the asset. The amount of impairment is measured as the difference between the asset’s estimated fair value and its book value. There were no triggering events requiring assessment of impairment as of March 31, 2021 and December 31, 2020. For the three months ended March 31, 2021 and 2020, no impairment of long-lived assets was recognized.
F-11
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Value Added Tax
The Company is subject to value added tax (“VAT”) on revenues earned for services provided in the PRC. The amount of VAT liability is determined by applying the applicable tax rates to the invoiced amount of services provided (output VAT) less VAT paid on purchases made with the relevant supporting invoices (input VAT). The Company reports revenue net of PRC’s value added tax for all the periods presented in the consolidated statements of operations and comprehensive loss.
Government Grants and Deferred Grant Income
Government grants related to purchased long-live assets, most of which are laboratory equipment for research and development, are recorded as deferred grant income initially and recognized as grant income on a systematic basis over the useful lives of the assets. Government grants that are to compensate incurred costs, expenses or losses are recognized in the current period. The Company applies the presentation method consistently to the similar government grants in the condensed consolidated financial statements. Government grants that are related to operating activities are included in operating income (loss), otherwise, they are recorded in other income (expense).
Grants and subsidies received from Chinese government are recognized when the proceeds are received or collectible and related milestones have been reached and all contingencies have been resolved. For the three months ended March 31, 2021 and 2020, grant income amounted to $123,467 and $526,703, respectively.
Deferred grant income represents grants collected but not earned as of each of the balance sheet date. This is primarily composed of receipts of the government subsidies for acquisition of lab equipment and reimbursement on office rent and research and development expense. As of March 31, 2021 and December 31, 2020, deferred grant income amounted to $488,113 and $612,356, respectively.
Accrued Liabilities and Other Payables
Accrued liabilities and other payables primarily consist of accrued and unpaid interest related to notes payable, taxes payable, and accrued liabilities for other miscellaneous items. As of March 31, 2021 and December 31, 2020, accrued liabilities and other payables amounted to $62,938 and $37,432, respectively.
Deferred Revenue
Payments received prior to services being performed are recorded as deferred revenue until such time as the services are performed. As of March 31, 2021 and December 31, 2020, deferred revenue amounted to $167,201 and $88,508, respectively.
Revenue Recognition
The Company recognizes revenue under Accounting Standards Codification (“ASC”) Topic 606, Revenue from Contracts with Customers (“ASC 606”). The core principle of the revenue standard is that a company should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. The following five steps are applied to achieve that core principle:
| ● | Step 1: Identify the contract with the customer |
| ● | Step 2: Identify the performance obligations in the contract |
| ● | Step 3: Determine the transaction price |
| ● | Step 4: Allocate the transaction price to the performance obligations in the contract |
| ● | Step 5: Recognize revenue when the company satisfies a performance obligation |
F-12
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Revenue Recognition (continued)
In order to identify the performance obligations in a contract with a customer, a company must assess the promised goods or services in the contract and identify each promised goods or service that is distinct. A performance obligation meets ASC 606’s definition of a “distinct” goods or service (or bundle of goods or services) if both of the following criteria are met:
| ● | The customer can benefit from the goods or service either on its own or together with other resources that are readily available to the customer (i.e., the goods or service is capable of being distinct). |
| ● | The entity’s promise to transfer the goods or service to the customer is separately identifiable from other promises in the contract (i.e., the promise to transfer the goods or service is distinct within the context of the contract). |
If a goods or service is not distinct, the goods or service is combined with other promised goods or services until a bundle of goods or services is identified that is distinct.
The transaction price is the amount of consideration to which an entity expects to be entitled in exchange for transferring promised goods or services to a customer, excluding amounts collected on behalf of third parties (for example, some sales taxes). The consideration promised in a contract with a customer may include fixed amounts, variable amounts, or both. Variable consideration is included in the transaction price only to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. From time to time, the variable consideration may include fees for services performed. The Company uses the expected value method to estimate the amount of variable consideration to be included in the transaction price.
The transaction price is allocated to each performance obligation on a relative standalone selling price basis. The transaction price allocated to each performance obligation is recognized when that performance obligation is satisfied, at a point in time or over time as appropriate.
The Company’s revenues are derived from providing general laboratory testing services and immunology and hematology testing services for patients and other customers. Revenues related to its service offerings are recognized at a point in time when service is rendered. Any payments received in advance of the performance of services are recorded as deferred revenue until such time as the services are performed. Except for deferred revenue, the Company did not have any other contract liability nor contract asset as of March 31, 2021 and December 31, 2020.
The Company does not offer promotional payments, customer coupons, rebates or other cash redemption offers to its customers.
The Company has concluded that its government grants are not within the scope of ASC Topic 606 as they do not meet the definition of a contract with a customer. The Company has concluded that the grants meet the definition of a contribution and are non-reciprocal transactions, and has also concluded that Subtopic 958-605, Not-for-Profit-Entities-Revenue Recognition does not apply, as it is a business entity and the grants are with governmental agencies.
In the absence of applicable guidance under US GAAP, effective January 1, 2018, the Company developed a policy for the recognition of grant revenue when the related costs are incurred or the related long-live assets are purchased, and the right to payment is realized.
The Company believes this policy is consistent with the overarching premise in ASC Topic 606, to ensure that revenue recognition reflects the transfer of promised goods or services to customers in an amount that reflects the consideration that it expects to be entitled to in exchange for those goods or services, even though there is no exchange as defined in ASC Topic 606.
The Company believes the recognition of revenue as costs are incurred or long-live assets are purchased, and amounts become realizable is analogous to the concept of transfer of control of a service over time under ASC Topic 606.
F-13
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Disaggregation of Revenues
In the following table, revenues are disaggregated by segment for the three months ended March 31, 2021 and 2020:
| For the three months ended March 31, | ||||||||||
| Revenue Stream | 2021 | 2020 | Revenue Stream Detail | |||||||
| General laboratory testing | $ | 945,648 | $ | 42,887 | Providing general laboratory testing services, including genomics, proteomics, routine blood/urine testing, COVID-19 PCR/antibody testing etc., to patients and other customers. | |||||
| Immunology and hematology testing | 301,857 | - | Providing immunology and hematology testing services, mainly related to cell therapy, including tumor biomarkers and immunophenotyping, to customers. | |||||||
| Total revenue | $ | 1,247,505 | $ | 42,887 | ||||||
Operating Lease
The Company determines if an arrangement contains a lease at the inception of a contract. Right-of-use assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising from the lease. Right-of- use assets and lease liabilities are recognized at the commencement date based on the present value of the remaining future minimum lease payments. As the interest rate implicit in the Company’s leases is not readily determinable, the Company utilizes its borrowing rates set by the Central Bank of the People’s Republic of China, determined by class of underlying asset, to discount the lease payments.
The Company leases premises for offices under non-cancellable operating leases. Operating lease payments are expensed over the term of lease. The Company leases do not include options to extend nor any restrictions or covenants. The Company has historically been able to renew its office leases. Under the terms of the lease agreements, the Company has no legal or contractual asset retirement obligations at the end of the lease.
Income Taxes
The Company accounts for income taxes using the asset/liability method prescribed by ASC 740, “Income Taxes.” Under this method, deferred tax assets and liabilities are determined based on the difference between the financial reporting and tax bases of assets and liabilities using enacted tax rates that will be in effect in the period in which the differences are expected to reverse. The Company records a valuation allowance to offset deferred tax assets if, based on the weight of available evidence, it is more-likely-than-not that some portion, or all, of the deferred tax assets will not be realized. The effect on deferred taxes of a change in tax rates is recognized as income or loss in the period that includes the enactment date.
The Company follows the accounting guidance for uncertainty in income taxes using the provisions of ASC 740 “Income Taxes”. Using that guidance, tax positions initially need to be recognized in the financial statements when it is more likely than not the position will be sustained upon examination by the tax authorities. As of March 31, 2021 and December 31, 2020, the Company had no significant uncertain tax positions that qualify for either recognition or disclosure in the financial statements. Tax year that remains subject to examination is the years ended December 31, 2017 through December 31, 2020. The Company recognizes interest and penalties related to significant uncertain income tax positions in income tax expense. However, no such interest and penalties were recorded as of March 31, 2021 and December 31, 2020.
F-14
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Loss per share
The Company computes loss per share in accordance with ASC 260, “Earnings per Share” (“ASC 260”). ASC 260 requires companies with complex capital structures to present basic and diluted earnings per share (“EPS”). Basic EPS is measured as net income divided by the weighted average common shares outstanding for the period. Diluted EPS presents the dilutive effect on a per share basis of potential common shares (e.g. convertible securities, options and warrants) as if they had been converted at the beginning of the periods presented, or issuance date, if later. Potential common shares that have an anti-dilutive effect (i.e. those that increase income per share or decrease loss per share) are excluded from the calculation of diluted EPS. As of March 31, 2021 and 2020, there were no dilutive shares.
Foreign Currency Translation
The reporting currency of the Company is U.S. Dollars. The functional currency of the parent company, Senlang, and Senlang HK, is the U.S. dollar and the functional currency of Senlang BJ, SenlangBio, and SenlangBio Clinical Laboratory is the Chinese Renminbi (“RMB”).
Monetary assets and liabilities denominated in currencies other than the reporting currency are translated into the reporting currency at the rates of exchange prevailing at the balance sheet date. Revenue and expenses are translated using average rates during each reporting period, and shareholders’ equity is translated at historical exchange rates. Cash flows are also translated at average translation rates for the periods, therefore, amounts reported on the statement of cash flows will not necessarily agree with changes in the corresponding balances on the consolidated balance sheet. Translation adjustments resulting from the process of translating the local currency financial statements into U.S. dollars are included in determining comprehensive income/loss. Transactions denominated in foreign currencies are translated into the functional currency at the exchange rates prevailing on the transaction dates. Assets and liabilities denominated in foreign currencies are translated into the functional currency at the exchange rates prevailing at the balance sheet date with any transaction gains and losses that arise from exchange rate fluctuations on transactions denominated in a currency other than the functional currency are included in the results of operations as incurred.
All of the Company’s revenue transactions are transacted in the functional currency of the Company. The Company does not enter into any material transaction in foreign currencies. Transaction gains or losses have not had, and are not expected to have, a material effect on the results of operations of the Company.
Asset and liability accounts at March 31, 2021 and December 31, 2020 were translated at 6.5527 RMB and 6.5306 RMB to $1.00, respectively, which were the exchange rates on the balance sheet dates. Equity accounts were stated at their historical rates. The average translation rates applied to the statements of operations for the three months ended March 31, 2021 and 2020 were 6.4843 RMB and 6.9823 RMB to $1.00, respectively. Cash flows from the Company’s operations are calculated based upon the local currencies using the average translation rate.
Commitment and Contingencies
In the normal course of business, the Company is subject to contingencies, such as legal proceedings and claims arising out of its business, that cover a wide range of matters. Liabilities for such contingencies are recorded when it is probable that a liability has been incurred and the amount of the assessment can be reasonably estimated.
Segment Reporting
The Company uses “the management approach” in determining reportable operating segments. The management approach considers the internal organization and reporting used by the Company’s chief operating decision maker for making operating decisions and assessing performance as the source for determining the Company’s reportable segments. The Company’s chief operating decision maker is its Chief Executive Officer (“CEO”), who reviews operating results to make decisions about allocating resources and assessing performance for the entire Company. The Company has determined that it has two reportable business segments: general laboratory testing segment, and immunology and hematology testing segment. These reportable segments offer different types of services and products, have different types of revenue, and are managed separately as each requires different operating strategies and management expertise.
Related Parties
Parties are considered to be related to the Company if the parties, directly or indirectly, through one or more intermediaries, control, are controlled by, or are under common control with the Company. Related parties also include principal owners of the Company, its management, members of the immediate families of principal owners of the Company and its management and other parties with which the Company may deal with if one party controls or can significantly influence the management or operating policies of the other to an extent that one of the transacting parties might be prevented from fully pursuing its own separate interests. The Company discloses all significant related party transactions.
F-15
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Recent Accounting Standards
In August 2018, the FASB issued ASU No. 2018-13, Fair Value Measurement (Topic 820): Disclosure Framework-Changes to the Disclosure Requirements for Fair Value Measurement. The objective of ASU 2018-13 is to improve the effectiveness of disclosures in the notes to the financial statements by removing, modifying, and adding certain fair value disclosure requirements to facilitate clear communication of the information required by generally accepted accounting principles. The amendments are effective for all entities for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2019 with early adoption permitted upon issuance of this ASU. The adoption of ASU 2018 – 13 did not have a material impact on the Company’s condensed consolidated financial statements.
In June 2016, the FASB issued ASU 2016-13, Financial Instruments - Credit Losses (“Topic 326”). The ASU introduces a new accounting model, the Current Expected Credit Losses model (“CECL”), which requires earlier recognition of credit losses and additional disclosures related to credit risk. The CECL model utilizes a lifetime expected credit loss measurement objective for the recognition of credit losses at the time the financial asset is originated or acquired. ASU 2016-13 is effective for annual period beginning after December 15, 2022, including interim reporting periods within those annual reporting periods. The Company expects that the adoption will not have a material impact on the Company’s condensed consolidated financial statements.
In December 2019, the FASB issued ASU 2019-12, Simplifying the Accounting for Income Taxes, as part of its Simplification Initiative to reduce the cost and complexity in accounting for income taxes. This standard removes certain exceptions related to the approach for intra period tax allocation, the methodology for calculating income taxes in an interim period and the recognition of deferred tax liabilities for outside basis differences. It also amends other aspects of the guidance to help simplify and promote consistent application of GAAP. The guidance is effective for interim and annual periods beginning after December 15, 2020, with early adoption permitted. The adoption of ASU 2019-12 did not have a material impact on the Company’s consolidated financial statements.
Other accounting standards that have been issued or proposed by FASB that do not require adoption until a future date are not expected to have a material impact on the consolidated financial statements upon adoption. The Company does not discuss recent pronouncements that are not anticipated to have an impact on or are unrelated to its consolidated financial condition, results of operations, cash flows or disclosures.
NOTE 4 – VARIABLE INTEREST ENTITY AND OTHER CONSOLIDATION MATTERS
On April 26, 2021, Senlang BJ entered into VIE Agreements with SenlangBio and 13 shareholders of SenlangBio. The key terms of these VIE Agreements are summarized in “NOTE 1 – ORGANIZATION AND NATURE OF OPERATIONS” above. As a result of the VIE Agreements, the Company classifies SenlangBio as a VIE.
A VIE is an entity that has either a total equity investment that is insufficient to permit the entity to finance its activities without additional subordinated financial support, or whose equity investors lack the characteristics of a controlling financial interest, such as through voting rights, right to receive the expected residual returns of the entity or obligation to absorb the expected losses of the entity. The variable interest holder, if any, that has a controlling financial interest in a VIE is deemed to be the primary beneficiary and must consolidate the VIE. Senlang BJ is deemed to have a controlling financial interest and be the primary beneficiary of SenlangBio, because it has both of the following characteristics:
| 1. | Power to direct activities of a VIE that most significantly impact the entity’s economic performance, and |
| 2. | Obligation to absorb losses of the entity that could potentially be significant to the VIE or right to receive benefits from the entity that could potentially be significant to the VIE. |
Pursuant to the VIE Agreements, SenlangBio pays service fees equal to all of its net income to Senlang BJ. At the same time, Senlang BJ is entitled to receive all of SenlangBio’s expected residual returns. The VIE Agreements are designed so that SenlangBio operates for the benefit of the Company. Accordingly, the accounts of SenlangBio are consolidated in the accompanying financial statements pursuant to ASC 810-10, Consolidation. In addition, its financial positions and results of operations are included in the Company’s consolidated financial statements.
F-16
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 4 – VARIABLE INTEREST ENTITY AND OTHER CONSOLIDATION MATTERS (continued)
In addition, as all of these VIE agreements are governed by PRC law and provide for the resolution of disputes through arbitration in the PRC, they would be interpreted in accordance with PRC law and any disputes would be resolved in accordance with PRC legal procedures. The legal environment in the PRC is not as developed as in other jurisdictions, such as the United States. As a result, uncertainties in the PRC legal system could further limit the Company’s ability to enforce these VIE agreements. Furthermore, these contracts may not be enforceable in China if PRC government authorities or courts take a view that such contracts contravene PRC laws and regulations or are otherwise not enforceable for public policy reasons. In the event the Company is unable to enforce these VIE agreements, it may not be able to exert effective control over SenlangBio and its ability to conduct its business may be materially and adversely affected.
All of the Company’s main current operations are conducted through SenlangBio and subsidiary of SenlangBio. Current regulations in China permit SenlangBio to pay dividends to the Company only out of its accumulated distributable profits, if any, determined in accordance with its article of association and PRC accounting standards and regulations. The ability of SenlangBio to make dividends and other payments to the Company may be restricted by factors including changes in applicable foreign exchange and other laws and regulations.
The following consolidated financial information of the VIE and VIE’s subsidiary as a whole as of March 31, 2021 and December 31, 2020 and for the three months ended March 31, 2021 and 2020 was included in the accompanying condensed consolidated financial statements of the Company. Transactions between the VIE and VIE’s subsidiary are eliminated in the financial information presented below:
| March 31, 2021 | December 31, 2020 | |||||||
| Cash | $ | 188,887 | $ | 18,935 | ||||
| Accounts receivable | 636,746 | 45,271 | ||||||
| Recoverable VAT | 343,755 | 335,150 | ||||||
| Inventory | 105,743 | 125,962 | ||||||
| Prepaid expenses and other current assets | 60,949 | 21,017 | ||||||
| Security deposit | 49,843 | 50,012 | ||||||
| Operating lease right-of-use assets, net | 266,406 | 320,123 | ||||||
| Property and equipment, net | 2,268,076 | 2,567,522 | ||||||
| Total Assets | 3,920,405 | 3,483,992 | ||||||
| Notes payable | 1,373,480 | 918,752 | ||||||
| Notes payable - related party | 244,174 | 245,000 | ||||||
| Accounts payable | 564,192 | 310,330 | ||||||
| Salary payable | 102,329 | 105,810 | ||||||
| Accrued leasehold improvements liabilities | 261,106 | 315,583 | ||||||
| Accrued liabilities and other payables | 62,938 | 37,432 | ||||||
| Deferred revenue | 167,201 | 88,508 | ||||||
| Deferred grant income | 183,496 | 260,679 | ||||||
| Operating lease obligation | 151,167 | 155,470 | ||||||
| Deferred grant income - noncurrent portion | 304,617 | 351,677 | ||||||
| Operating lease obligation - noncurrent portion | 52,377 | 105,566 | ||||||
| Total Liabilities | 3,467,077 | 2,894,807 | ||||||
| Total shareholders’ equity | $ | 453,328 | $ | 589,185 | ||||
F-17
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 4 – VARIABLE INTEREST ENTITY AND OTHER CONSOLIDATION MATTERS (continued)
| For the Three Months Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| Revenues | $ | 1,247,505 | $ | 42,887 | ||||
| Loss from operations | (90,663 | ) | (208,256 | ) | ||||
| Net loss | $ | (135,280 | ) | $ | (208,672 | ) | ||
NOTE 5 – PROPERTY AND EQUIPMENT, NET
At March 31, 2021 and December 31, 2020, property and equipment consisted of the following:
| March 31, 2021 | December 31, 2020 | |||||||
| Laboratory equipment | $ | 2,261,276 | $ | 2,268,929 | ||||
| Electronic equipment | 235,628 | 234,212 | ||||||
| Tools and furniture | 199,053 | 198,361 | ||||||
| Vehicles | 63,104 | 63,317 | ||||||
| Leasehold improvements | 2,244,852 | 2,252,449 | ||||||
| Software | 26,737 | 26,858 | ||||||
| Total | 5,030,650 | 5,044,126 | ||||||
| Less: accumulated depreciation | (2,762,574 | ) | (2,476,604 | ) | ||||
| Property and equipment, net | $ | 2,268,076 | $ | 2,567,522 | ||||
For the three months ended March 31, 2021 and 2020, depreciation expense of property and equipment amounted to $297,458 and $252,099, respectively, of which, $14,586 and $0 was included in cost of revenue, $96,998 and $107,137 was included in research and development expenses, and $185,874 and $144,962 was included in general and administrative expense, respectively.
NOTE 6 – NOTES PAYABLE
From time to time, the Company acquires loans from various entities to fund its operations. These loans are due within one year and are unsecured and uncollateralized. At March 31, 2021 and December 31, 2020, short-term borrowings consisted of the following:
| March 31, 2021 | December 31, 2020 | |||||||
| Loan from China Construction Bank, due on February 17, 2021 with annual interest rate of 4.1025%, extended to May 17, 2021 with annual interest rate of 3.8525% and repaid on the extended date (See Note 14) | $ | 457,827 | $ | 459,376 | ||||
| Loan from a third-party company, due on February 8, 2021 with annual interest rate of 4.35%, extended through October 2021 | 457,827 | 459,376 | ||||||
| Loan from a third-party company, due on October 31, 2021 with annual interest rate of 4.35% | 457,826 | - | ||||||
| Total | $ | 1,373,480 | $ | 918,752 | ||||
For the three months ended March 31, 2021, interest expense related to borrowings amounted to $13,647 and have been included in interest expense on the accompanying unaudited condensed consolidated statements of operations and comprehensive loss. The Company had neither borrowing activity nor interest expense in the first quarter of 2020.
As of March 31, 2021 and December 31, 2020, the related accrued and unpaid interest for borrowings was $8,907 and $2,942, respectively, and have been included in accrued liabilities and other payables on the accompanying condensed consolidated balance sheets.
F-18
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 7 – RELATED PARTY TRANSACTIONS
Notes Payable - Related Party
From time to time, the Company acquires loans from Hebei Senlang Taihe Biological Technology Co. Ltd. (“Taihe”) to fund its operations. These loans are due within one year and are unsecured and uncollateralized. The annual interest rate for these loans is 4.35%. SenlangBio’s largest shareholder is the former executive director and currently holds 80% of equity interest of Taihe.
As of March 31, 2021 and December 31, 2020, the outstanding principal amounted to $244,174 and $245,000, respectively, and were recorded as “Notes payable – related party” on the accompanying condensed consolidated balance sheets.
For the three months ended March 31, 2021 and 2020, interest expense related to related party borrowings amounted to $2,683 and $1,860, respectively, and have been included in interest expense – related party on the accompanying condensed consolidated statements of operations and comprehensive loss. As of both March 31, 2021 and December 31, 2020, the related interest for related party borrowings was paid in full.
NOTE 8 – INCOME TAXES
British Virgin Islands
Under the current laws of BVI, Senlang is not subject to tax on income or capital gain. In addition, payments of dividends by the Company to its shareholders are not subject to withholding tax in the BVI.
Hong Kong
Senlang HK is incorporated in Hong Kong and is subject to Hong Kong Profits Tax on the taxable income as reported in its statutory financial statements adjusted in accordance with relevant Hong Kong tax laws. The applicable tax rate is 16.5% on its taxable income generated from operations in Hong Kong. The Company did not make any provisions for Hong Kong profit tax as there were no assessable profits derived from or earned in Hong Kong since inception. Additionally, payments of dividends by the subsidiary incorporated in Hong Kong to the Company are not subject to any Hong Kong withholding tax.
United States
The Company and its subsidiaries have no presence in the United States and does not conduct business in the United States, therefore no United States income tax should be imposed upon the Company and its subsidiaries.
PRC
Senlang BJ, SenlangBio and SenlangBio Clinical Laboratory are subject to PRC Enterprise Income Tax (“EIT”) on the taxable income in accordance with the relevant PRC income tax laws. The EIT rate for companies operating in the PRC is 25%. In the year ended December 2020, Senlang BJ, SenlangBio and SenlangBio Clinical Laboratory were each recognized as small low-profit enterprise and received a preferential income tax rate of 10%. In the year ending December 2021, Senlang BJ, SenlangBio and SenlangBio Clinical Laboratory are each expected to be recognized as small low-profit enterprise and are expected to receive a preferential income tax rate of 10%. The Company did not have any income taxes expense for the years ended December 31, 2020 since it incurred losses in the periods. As of March 31, 2021, income tax returns for the tax years ended December 31, 2017 through December 31, 2020 remain open for statutory examination by PRC tax authorities.
Below is a reconciliation of the statutory tax rate to the effective tax rate for the three months ended March 31, 2021 and 2020:
| For the three months ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| PRC statutory income tax rate | 25.0 | % | 25.0 | % | ||||
| Effect of income tax exemptions and reliefs | (15.0 | )% | (15.0 | )% | ||||
| Effect of non-deductible expense | 0.1 | % | 0.1 | % | ||||
| Valuation allowance | (36.8 | )% | (10.1 | )% | ||||
| Effective tax rate | (26.7 | )% | 0.0 | % | ||||
The Company’s approximate net deferred tax assets as of March 31, 2021 and December 31, 2020 were as follows:
| Deferred tax assets: | March 31, 2021 | December 31, 2020 | ||||||
| Net operating loss carryforward | $ | 1,426,050 | $ | 1,340,709 | ||||
| Valuation allowance | (1,426,050 | ) | (1,340,709 | ) | ||||
| Net deferred tax assets | $ | - | $ | - | ||||
F-19
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 8 – INCOME TAXES (continued)
PRC (continued)
The Company provided a valuation allowance equal to the deferred income tax assets as of March 31, 2021 and December 31, 2020 because it was not known whether future taxable income will be sufficient to utilize the loss carryforward. The potential tax benefit arising from the loss carryforward will begin to expire in 2023.
As of March 31, 2021 and December 31, 2020, the Company had no significant uncertain tax positions that qualify for either recognition or disclosure in the financial statements. As of March 31, 2021, income tax returns for the tax years ended December 31, 2017 through December 31, 2020 remain open for statutory examination by PRC tax authorities.
The uncertain tax positions are related to tax years that remain subject to examination by the relevant tax authorities. Based on the outcome of any future examinations, or as a result of the expiration of statute of limitations for specific jurisdictions, it is reasonably possible that the related unrecognized tax benefits for tax positions taken regarding previously filed tax returns, might materially change from those recorded as liabilities for uncertain tax positions in the Company’s consolidated financial statements as of March 31, 2021 and December 31, 2020. In addition, the outcome of these examinations may impact the valuation of certain deferred tax assets (such as net operating losses) in future periods. The Company’s policy is to recognize interest and penalties accrued on any unrecognized tax benefits, if any, as a component of income tax expense. The Company does not anticipate any significant increases or decreases to its liability for unrecognized tax benefit within the next twelve months.
According to the PRC Tax Administration and Collection Law, the statute of limitations is three years if the underpayment of income taxes is due to computational errors made by the taxpayer. The statute of limitations will be extended to five years under special circumstances, which are not clearly defined, but an underpayment of income tax liability exceeding RMB100,000 (approximately $15,000) is specifically listed as a special circumstance. In the case of a transfer pricing related adjustment, the statute of limitations is ten years. There is no statute of limitations in the case of tax evasion.
Accounting for Uncertainty in Income Taxes
The tax authority of the PRC government conducts periodic and ad hoc tax filing reviews on business enterprises operating in the PRC after those enterprises complete their relevant tax filings. Therefore, the Company’s tax filings results are subject to change. It is therefore uncertain as to whether the PRC tax authority may take different views about the Company’s tax filings, which may lead to additional tax liabilities.
ASC 740 requires recognition and measurement of uncertain income tax positions using a “more-likely-than-not” approach. The management evaluated the Company’s tax positions and concluded that no provision for uncertainty in income taxes was necessary as of December 31, 2020 and 2019.
NOTE 9 – EQUITY
The equity structures as of March 31, 2021 was presented after giving retroactive effect to the reorganization of the Company that was completed in April 2021. Immediately before and after the reorganization, the shareholders of SenlangBio controlled Senlang. Therefore, the reorganization is accounted for as a transaction of entities under common control at carrying value and for accounting purpose, the reorganization was accounted for as a recapitalization. The consolidated financial statements are prepared on the basis as if the Reorganization became effective as of the beginning of the first period presented in the accompanying consolidated financial statements of the Company.
Ordinary Shares
On October 15, 2020, Senlang was incorporated in the British Virgin Islands. As of the date of this report, its authorized share capital consists of 50,000 ordinary shares with a par value of $1.00 per share. As of the date of this report, 10,001 ordinary shares were issued and outstanding.
Statutory Reserve
Senlang BJ, SenlangBio and SenlangBio Clinical Laboratory operate in the PRC and are required to reserve 10% of their net profit after income tax, as determined in accordance with the PRC accounting rules and regulations. Appropriation to the statutory reserve by the Company is based on profit arrived at under PRC accounting standards for business enterprises for each year.
The profit arrived at must be set off against any accumulated losses sustained by the Company in prior years, before allocation is made to the statutory reserve. Appropriation to the statutory reserve must be made before distribution of dividends to shareholders. The appropriation is required until the statutory reserve reaches 50% of the registered capital. This statutory reserve is not distributable in the form of cash dividends. Senlang BJ, SenlangBio and SenlangBio Clinical Laboratory did not make any appropriation to statutory reserve during the three months ended March 31, 2021 and 2020. As of both March 31, 2021 and December 31, 2020, the Company did not have any statutory reserve.
F-20
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 10 - CONCENTRATIONS
Customers
The following table sets forth information as to each customer that accounted for 10% or more of the Company’s revenues for the three months ended March 31, 2021 and 2020.
| Three Months Ended March 31, | ||||||||
| Customer | 2021 | 2020 | ||||||
| A | 43 | % | * | |||||
| B | 13 | % | * | |||||
| C | * | 20 | % | |||||
| D | * | 10 | % | |||||
| E | * | 10 | % | |||||
| * | Less than 10% |
Two customers, whose outstanding receivable accounted for 10% or more of the Company’s total outstanding accounts receivable at March 31, 2021, accounted for 97% of the Company’s total outstanding accounts receivable at March 31, 2021.
One customer, whose outstanding receivable accounted for 10% or more of the Company’s total outstanding accounts receivable at December 31, 2020, accounted for 98.1% of the Company’s total outstanding accounts receivable at December 31, 2020.
Suppliers
The following table sets forth information as to each supplier that accounted for 10% or more of the Company’s purchase for the three months ended March 31, 2021 and 2020.
| Three Months Ended March 31, | ||||||||
| Supplier | 2021 | 2020 | ||||||
| A | 40 | % | * | |||||
| B | 19 | % | * | |||||
| C | * | 20 | % | |||||
| D | * | 10 | % | |||||
| * | Less than 10% |
Two suppliers, whose outstanding payable accounted for 10% or more of the Company’s total outstanding accounts payable at March 31, 2021, accounted for 35.7% of the Company’s total outstanding accounts payable at March 31, 2021.
Three suppliers, whose outstanding payable accounted for 10% or more of the Company’s total outstanding accounts payable at December 31, 2020, accounted for 77.8% of the Company’s total outstanding accounts payable at December 31, 2020.
NOTE 11 – SEGMENT INFORMATION
For the three months ended March 31, 2021 and 2020, the Company operated in two reportable business segments - (1) general laboratory testing segment, and (2) immunology and hematology testing segment. The Company’s reportable segments are strategic business units that offer different services and products. They are managed separately based on the fundamental differences in their operations. Information with respect to these reportable business segments for the three months ended March 31, 2021 and 2020 was as follows:
F-21
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 11 – SEGMENT INFORMATION (continued)
| Three Months Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| Revenues | ||||||||
| General laboratory testing | $ | 945,648 | $ | 42,887 | ||||
| Immunology and hematology testing | 301,857 | - | ||||||
| Total | 1,247,505 | 42,887 | ||||||
| Cost of revenues | ||||||||
| General laboratory testing | 347,911 | 14,117 | ||||||
| Immunology and hematology testing | 89,498 | - | ||||||
| Total | 437,409 | 14,117 | ||||||
| Gross profit | ||||||||
| General laboratory testing | 597,737 | 28,770 | ||||||
| Immunology and hematology testing | 212,359 | - | ||||||
| Total | 810,096 | 28,770 | ||||||
| Operating expenses | ||||||||
| General laboratory testing | 59,480 | 50,969 | ||||||
| Immunology and hematology testing | 841,279 | 186,057 | ||||||
| Total | 900,759 | 237,026 | ||||||
| Other (expense) income | ||||||||
| Interest expense | ||||||||
| Immunology and hematology testing | (16,330 | ) | (1,860 | ) | ||||
| Other income | ||||||||
| General laboratory testing | 96 | 1,072 | ||||||
| Immunology and hematology testing | 130 | 372 | ||||||
| Total | 226 | 1,444 | ||||||
| Total other expense, net | (16,104 | ) | (416 | ) | ||||
| Income taxes | ||||||||
| General laboratory testing | 28,513 | - | ||||||
| Immunology and hematology testing | - | - | ||||||
| Total | 28,513 | - | ||||||
| Net income (loss) | ||||||||
| General laboratory testing | 509,840 | (21,127 | ) | |||||
| Immunology and hematology testing | (645,120 | ) | (187,545 | ) | ||||
| Total | (135,280 | ) | (208,672 | ) | ||||
| Depreciation | ||||||||
| General laboratory testing | 3,970 | 3,456 | ||||||
| Immunology and hematology testing | 293,488 | 248,643 | ||||||
| Total | 297,458 | 252,099 | ||||||
| Capital expenditure | ||||||||
| General laboratory testing | 1,376 | 6,874 | ||||||
| Immunology and hematology testing | 56,205 | 11,791 | ||||||
| Total | $ | 57,581 | $ | 18,665 | ||||
F-22
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 11 – SEGMENT INFORMATION (continued)
| Identifiable long-lived tangible assets at March 31, 2021 and December 31, 2020 | March 31, 2021 | December 31, 2020 | ||||||
| General laboratory testing | $ | 59,669 | $ | 62,447 | ||||
| Immunology and hematology testing | 2,208,407 | 2,505,075 | ||||||
| Total | 2,268,076 | 2,567,522 | ||||||
| Total assets at March 31, 2021 and December 31, 2020 | ||||||||
| General laboratory testing | 809,399 | 163,560 | ||||||
| Immunology and hematology testing | 3,111,006 | 3,320,432 | ||||||
| Total | $ | 3,920,405 | $ | 3,483,992 | ||||
The Company does not have long-lived assets located outside the PRC.
NOTE 12 – COMMITMENTS AND CONTINCENGIES
Contingencies
From time to time, the Company may be subject to certain legal proceedings, claims and disputes that arise in the ordinary course of business. Although the outcomes of these legal proceedings cannot be predicted, the Company does not believe these actions, in the aggregate, will have a material adverse impact on its financial position, results of operations or liquidity.
Operating Leases Commitment
The Company is a party to leases for office space. Rent expense under all operating leases amounted to approximately $55,000 and $54,000 for the three months ended March 31, 2021 and 2020, respectively.
Supplemental cash flow information related to leases for the three months ended March 31, 2021 and 2020 is as follows:
| Three Months Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| Cash paid for amounts included in the measurement of lease liabilities: | ||||||||
| Operating cash flows paid for operating lease | $ | 59,039 | $ | 54,687 | ||||
| Right-of-use assets obtained in exchange for lease obligation: | ||||||||
| Operating lease | $ | - | $ | - | ||||
The following table summarizes the lease term and discount rate for the Company’s operating lease as of March 31, 2021:
| Operating Lease | ||||
| Weighted average remaining lease term (in years) | 1.02 | |||
| Weighted average discount rate | 4.75 | % | ||
F-23
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 12 – COMMITMENTS AND CONTINCENGIES (continued)
Operating Leases Commitment (continued)
The following table summarizes the maturity of lease liabilities under operating lease as of March 31, 2021:
| For the Twelve-month Period Ending March 31: | Operating Lease | |||
| 2022 | $ | 162,681 | ||
| 2023 | 52,999 | |||
| 2024 and thereafter | - | |||
| Total lease payments | 215,680 | |||
| Amount of lease payments representing interest | (12,136 | ) | ||
| Total present value of operating lease liabilities | $ | 203,544 | ||
| Current portion | $ | 151,167 | ||
| Long-term portion | 52,377 | |||
| Total | $ | 203,544 | ||
Variable Interest Entity Structure
In the opinion of management, (i) the corporate structure of the Company is in compliance with existing PRC laws and regulations; (ii) the Contractual Arrangements are valid and binding, and do not result in any violation of PRC laws or regulations currently in effect; and (iii) the business operations of WFOE, VIE and VIE’s subsidiary are in compliance with existing PRC laws and regulations in all material respects.
However, there are substantial uncertainties regarding the interpretation and application of current and future PRC laws and regulations. Accordingly, the Company cannot be assured that PRC regulatory authorities will not ultimately take a contrary view to the foregoing opinion of its management. If the current corporate structure of the Company or the Contractual Arrangements is found to be in violation of any existing or future PRC laws and regulations, the Company may be required to restructure its corporate structure and operations in the PRC to comply with changing and new PRC laws and regulations. In the opinion of management, the likelihood of loss in respect of the Company’s current corporate structure or the VIE Agreements is remote based on current facts and circumstances.
NOTE 13 – RESTRICTED NET ASSETS
As of March 31, 2021, the Company’s operations are conducted through its PRC subsidiary, VIE and VIE’s subsidiary, which can only pay dividends out of their retained earnings determined in accordance with the accounting standards and regulations in the PRC and after they have met the PRC requirements for appropriation to statutory reserve. In addition, most of the Company’s businesses and assets are denominated in RMB, which is not freely convertible into foreign currencies. All foreign exchange transactions take place either through the People’s Bank of China or other banks authorized to buy and sell foreign currencies at the exchange rates quoted by the People’s Bank of China. Approval of foreign currency payments by the People’s Bank of China or other regulatory institutions requires submitting a payment application form together with suppliers’ invoices, shipping documents and signed contracts. These currency exchange control procedures imposed by the PRC government authorities may restrict the ability of the Company’s PRC subsidiary to transfer their net assets to the Lonlon Biotech Ltd. (the “Parent Company”) through loans, advances or cash dividends.
Schedule I of Article 5-04 of Regulation S-X requires the condensed financial information of the parent company to be filed when the restricted net assets of consolidated subsidiaries exceed 25 percent of consolidated net assets as of the end of the most recently completed fiscal year. For purposes of this test, restricted net assets of consolidated subsidiaries shall mean that amount of the registrant’s proportionate share of net assets of its consolidated subsidiaries (after intercompany eliminations) which as of the end of the most recent fiscal year may not be transferred to the parent company in the form of loans, advances or cash dividends without the consent of a third party. The restricted net assets of the Company’s PRC subsidiary amounted to approximately $453,000 and $589,000 as of March 31, 2021 and December 31, 2020, respectively.
F-24
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 13 – RESTRICTED NET ASSETS (continued)
The Company’s PRC subsidiary’ net assets as of March 31, 2021 and December 31, 2020 exceeded 25% of the Company’s consolidated net assets. Accordingly, Parent Company’s condensed financial statements have been prepared in accordance with Rule 5-04 and Rule 12-04 of SEC Regulation S-X, and are as follows.
Condensed Financial Information of the Parent Company
The Parent Company’s condensed financial statements have been prepared using the same accounting principles and policies described in the notes to the consolidated financial statements, with the only exception being that the Parent Company accounts for its subsidiaries using the equity method. Refer to the consolidated financial statements and notes presented above for additional information and disclosures with respect to these financial statements.
| Parent Company’s Condensed Balance Sheets | ||||||||
| March 31, 2021 | December 31, 2020 | |||||||
| ASSETS | ||||||||
| NON-CURRENT ASSETS: | ||||||||
| Investment in subsidiaries | $ | 453,328 | $ | 589,185 | ||||
| Total Non-current Assets | 453,328 | 589,185 | ||||||
| Total Assets | $ | 453,328 | $ | 589,185 | ||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
| Total Liabilities | $ | - | $ | - | ||||
| Commitments and Contingencies | ||||||||
| SHAREHOLDERS’ EQUITY: | ||||||||
Ordinary shares, $1.00 par value; 50,000 shares authorized; 10,001 shares issued and outstanding at March 31, 2021 and December 2020 * | 10,001 | 10,001 | ||||||
| Additional paid-in capital | 8,946,197 | 8,946,197 | ||||||
| Accumulative deficit | (8,515,294 | ) | (8,380,014 | ) | ||||
| Accumulated other comprehensive income | 12,424 | 13,001 | ||||||
| Total Shareholders’ Equity | 453,328 | 589,185 | ||||||
| Total Liabilities and Shareholders’ Equity | $ | 453,328 | $ | 589,185 | ||||
| * | The shares amounts are presented on a retroactive basis. |
| Parent Company’s Condensed Statements of Operations and Comprehensive Loss | ||||||||
| For the Three Months Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| Revenue | $ | - | $ | - | ||||
| Operating expense | - | - | ||||||
| Income attributable to Parent Company only | - | - | ||||||
| Share of loss from investment in subsidiaries | (135,280 | ) | (208,672 | ) | ||||
| Net loss | (135,280 | ) | (208,672 | ) | ||||
| Other comprehensive loss | ||||||||
| Unrealized foreign currency translation loss | (577 | ) | (47,085 | ) | ||||
| Comprehensive loss | $ | (135,857 | ) | $ | (255,757 | ) | ||
F-25
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 13 – RESTRICTED NET ASSETS (continued)
Condensed Financial Information of the Parent Company (continued)
| Parent Company’s Condensed Statements of Cash Flows | ||||||||
| For the Three Months Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | (135,280 | ) | $ | (208,672 | ) | ||
| Adjustments to reconcile net loss to net cash provided by operating activities: | ||||||||
| Share of loss from investment in subsidiaries | 135,280 | 208,672 | ||||||
| NET CASH PROVIDED BY OPERATING ACTIVITIES | - | - | ||||||
| NET INCREASE IN CASH | - | - | ||||||
| CASH - beginning of period | - | - | ||||||
| CASH - end of period | $ | - | $ | - | ||||
Basis of Preparation
The condensed financial information of the Parent Company has been prepared using the same accounting policies as set out in the consolidated financial statements except that the Company used the equity method to account for investment in its subsidiaries.
Certain information and footnote disclosures normally included in financial statements prepared in conformity with accounting principles generally accepted in the United States of America have been condensed or omitted. The Parent Company only financial information has been derived from the Company’s consolidated financial statements and should be read in conjunction with the Company’s consolidated financial statements.
Investment in Subsidiaries
The Company and its subsidiaries were included in the consolidated financial statements where the inter-company balances and transactions were eliminated upon consolidation. For purpose of the Parent Company’s stand-alone financial statements, its investments in subsidiaries were reported using the equity method of accounting. Such investment is presented as “Investment in subsidiaries” on the condensed balance sheets and the subsidiaries’ loss is presented as “Share of loss from investment in subsidiaries” in the condensed statements of operations.
NOTE 14 – SUBSEQUENT EVENTS
In May 2021, the Company repaid a short-term loan from China Construction Bank in the principal amount of $457,827.
In May 2021, the Company borrowed a short-term loan from a related party company in the principal amount of $763,044. The new loan bears interest rate at 4.35% per annum and is due on October 31, 2021.
On April 26, 2021, Senlang BJ entered into a series of contractual arrangements, or VIE agreements with SenlangBio and 13 equity holders of SenlangBio, through which the Company obtained control and became the primary beneficiary of SenlangBio. As a result, SenlangBio became the Company’s VIE.
On June 13, 2021, the Company entered into a Share Purchase Agreement (the “Purchase Agreement”), by and among the Company, Avalon GloboCare Corp., a Delaware corporation (“Avalon”), the holders of the share capital of Senlang (the “Senlang Shareholders”), the ultimate beneficial owners of the Senlang Shareholders (the “Senlang Beneficial Shareholders” and, together with the Senlang Shareholders, the “Senlang Owners”) and a representative of the Senlang Owners (the “Senlang Representative”). Pursuant to the Purchase Agreement, Avalon will acquire all of the issued and outstanding share capital of the Company in consideration of 81 million shares of the common stock, par value $0.0001 per share, of Avalon (the “Acquisition”).
In connection with the Acquisition, on June 13, 2021, an institutional investor (the “Investor”) entered into an agreement with the related to the purchase of registered capital of the SenlangBio (the “SenlangBio Capital Increase Agreement”) pursuant to which the Investor will acquire an aggregate of up to 13.5% of the equity ownership of the SenlangBio for an aggregate purchase price of RMB 200,000,000 (approximately $30 million) (the “Equity Financing”), which funds will be invested in the SenlangBio in three installments of RMB 67,000,000 (approximately $10 million), RMB 67,000,000 (approximately $10 million) and RMB 66,000,000 (approximately $10 million), the first to be upon the closing of the Acquisition, the second to be within three months after the closing and the third to be within six months after the closing. In addition, pursuant to a Securities Exchange Agreement (the “Exchange Agreement”), by and among the Company, SenlangBio, Avalon and the Investor, dated June 13, 2021, the Investor has the right, exercisable between the six-month and five year-anniversaries of the respective initial closing and installment closings, to elect to exchange, from time to time, all or part of its then-owned equity ownership of SenlangBio for shares (the “Exchange Shares”) of Avalon Common Stock at a fixed exchange price of US$1.21 per share of Avalon Common Stock, which was the market price of the Avalon Common Stock as of the date of the Exchange Agreement under Nasdaq rules. In addition, the Exchange Agreement provides that the Investor may only exchange up to 10% of its total investment amount in any 30-day period.
These unaudited condensed consolidated financial statements were approved by management and available for issuance on June 24, 2021. The Company evaluated subsequent events through the date these unaudited condensed consolidated financial statements were issued.
F-26