UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a) of the
Securities Exchange Act of 1934
Filed by the Registrant ☒ Filed by a Party other than the Registrant ☐
Check the appropriate box:
| ☒ | Preliminary Proxy Statement | |
| ☐ | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) | |
| ☐ | Definitive Proxy Statement | |
| ☐ | Definitive Additional Materials | |
| ☐ | Soliciting Material Pursuant to §240.14a-12 | |
AVALON GLOBOCARE CORP.
(Name of Registrant as Specified In Its Charter)
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)
Payment of Filing Fee (Check the appropriate box):
| ☐ | No fee required. | |||
| ☒ | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. | |||
| (1) | Title of each class of securities to which transaction applies: | |||
|
Common shares, $1.00 par value per share, of Lonlon Biotech Ltd. | ||||
| (2) | Aggregate number of securities to which transaction applies: | |||
|
10,001 shares, $1.00 par value, Lonlon Biotech Ltd. | ||||
| (3) |
Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): $0.33 per share, calculated in accordance with Rule 0-11(c)(1)(i) based on the value of the shares of Lonlon Biotech Ltd. being acquired by the registrant, who is the acquiring person, established in accordance with Rule 0-11(a)(4) for securities of issuers with an accumulated capital deficit based on one-third of the par value of such shares, or $3,333.67 in the aggregate. In accordance with Section 14(g) of the Securities Exchange Act of 1934, as amended, the filing fee was determined by multiplying the aggregate value calculated in the preceding sentence by $0.0001091. | |||
| (4) | Proposed maximum aggregate value of transaction: | |||
|
$3,333.67 | ||||
| (5) | Total fee paid: | |||
|
$0.37 | ||||
| ☐ | Fee paid previously with preliminary materials: | |||
| ☐ | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. | |||
| (1) | Amount Previously Paid: | |||
| (2) | Form, Schedule or Registration Statement No.: | |||
| (3) | Filing Party: | |||
| (4) | Date Filed: | |||
Persons who are to respond to the collection of information contained in this form are not required to respond unless the form displays a currently valid OMB control number.
PRELIMINARY PROXY STATEMENT—SUBJECT TO COMPLETION
DATED JULY 15, 2021
AVALON GLOBOCARE CORP.
4400 Route 9 South, Suite 3100
Freehold, New Jersey 07728
[_], 2021
Dear Stockholder:
You are cordially invited to attend the annual meeting of stockholders of Avalon GloboCare Corp., a Delaware corporation (“Avalon” or the “Company”), which will be held at [_] on [_], 2021, beginning at [_], local time (the “annual meeting”), for the purposes detailed below, including (i) the election of our directors as described herein, (ii) the ratification of the appointment of Marcum LLP as the Company’s independent auditors for the fiscal year ending December 31, 2021 as described herein and (iii) the approval of the issuance of the shares of common stock, par value US$0.0001 per share, of Avalon (the “Avalon Common Stock”) to be issued in connection with the proposed acquisition of Lonlon Biotech Ltd., a company incorporated in the British Virgin Islands (“BVI”) (“Sen Lang”) by Avalon and the related facilitating transactions, including the Equity Financing (as defined below), pursuant to the rules of the Nasdaq Stock Market (“Nasdaq”) as described herein.
As previously announced, on June 13, 2021, Avalon entered into a Share Purchase Agreement (the “Purchase Agreement”), by and among the Company, Sen Lang, the holders of the share capital of Sen Lang (the “Sen Lang Shareholders”), the ultimate beneficial owners of the Sen Lang Shareholders (the “Sen Lang Beneficial Shareholders” and, together with the Sen Lang Shareholders, the “Sen Lang Owners”) and a representative of the Sen Lang Owners (the “Sen Lang Representative”). Pursuant to the Purchase Agreement, subject to the satisfaction of the conditions to closing therein, including approval by the Avalon stockholders pursuant to the rules of Nasdaq, Avalon agreed to purchase (the “Acquisition”) all of the issued and outstanding share capital of Sen Lang (the “Sen Lang Shares”). A copy of the Purchase Agreement is attached as Annex A to the accompanying proxy statement, and you are encouraged to read it in its entirety.
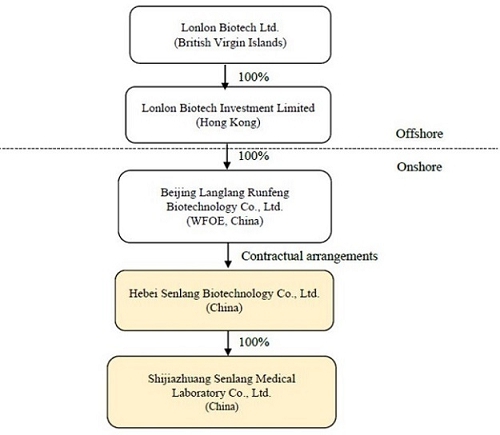
Sen Lang, through a “variable interest entity” (“VIE”) structure (described in more detail in the section of the accompanying proxy statement titled “The Acquisition—The VIE Structure”) of contractual rights held by its wholly-owned subsidiary Beijing Langlang Runfeng Biotechnology Co., Ltd., a wholly foreign owned enterprise with limited liability organized and existing under the laws of the People’s Republic of China (the “PRC”) (the “PRC Subsidiary”), has full economic benefit and management control over, and is consolidated for accounting purposes with, Senlang Biotechnology Co. Ltd., a PRC domestic company with limited liability organized and existing under the laws of the PRC (the “OpCo” or “SenlangBio”). SenlangBio is mainly engaged in the business of research and development in relation to CAR-T cell therapy, immune cell therapy and related drug development. SenlangBio is owned 100% by certain of the Sen Lang Beneficial Shareholders. A wholly-owned subsidiary of SenlangBio, Shijiazhuang Senlang Medical Laboratory Co., Ltd., a company with limited liability organized and existing under the laws of the PRC (“SenlangBio Clinical Laboratory”) is engaged in the business of testing of immunology, serology and molecular genetics specialties for patients, including hematology-tumor diagnostics and testing prior to clinical trials for cell therapy.
Prior to the execution of the Purchase Agreement, the Board of Directors of Avalon (the “Board”), unanimously (i) determined that the terms and provisions of the Purchase Agreement and the transactions contemplated thereby, including the Acquisition, are fair to, advisable and in the best interests of the Company and its stockholders, (ii) approved the Purchase Agreement and the transactions contemplated thereby, including the Acquisition and the issuance of the Acquisition Shares (as defined below), (iii) authorized, empowered and directed the Company to perform all of its obligations under the Purchase Agreement and the Exchange Agreement (as defined below) and related documents, and (iv) resolved to recommend the approval by the stockholders of the Company of the issuance of the Acquisition Shares in connection with the Acquisition and the issuance of the Exchange Shares (as defined below) in connection with the Exchange Agreement in compliance with the rules of Nasdaq (the “Company Board Recommendation”). Accordingly, the board recommends a vote “FOR” the proposal to approve the issuance of the Acquisition Shares in connection with the Acquisition and the issuance of the Exchange Shares in connection with the Exchange Agreement in compliance with the rules of Nasdaq and “FOR” each of the other proposals to be voted on at the annual meeting.
The purchase price being paid by Avalon to the Sen Lang Shareholders under the Purchase Agreement for the Sen Lang Shares is an aggregate of 81 million shares (the “Acquisition Shares”) of Avalon Common Stock. Ten percent (10%), or 8.1 million, of such shares will be held in escrow for 12 months following the closing to satisfy any indemnification obligations of the Sen Lang Shareholders under the Share Purchase Agreement. In addition, at the closing of the Acquisition, it is expected that Dr. Jianqiang Li, scientific founder and CSO of SenlangBio, will join the board of the Company, and Dr. Li will also be appointed as Chief Technology Officer of the Company. The Acquisition Shares will not be registered under the Securities Act of 1933, as amended (the “Securities Act”) and, therefore, will be restricted securities under Rule 144 under the Securities Act for six months or longer after the closing of the Acquisition, subject to “affiliate” status with the Company under the Securities Act.
In connection with the Acquisition, on June 13, 2021, an institutional investor (the “Investor”) entered into an agreement, as amended on June 24, 2021, with SenlangBio related to the purchase of registered capital of SenlangBio (the “OpCo Capital Increase Agreement”) pursuant to which the Investor will acquire an aggregate of up to 13.5% of the equity ownership of SenlangBio for an aggregate purchase price (the “Subscription Amount) of approximately US$30,000,000 (represented by an actual investment of RMB200,000,000) (the “Equity Financing”), which funds will be invested in SenlangBio in three equal installments of approximately US$10,000,000, at a fixed price, the first to be upon the closing of the Acquisition, the second to be within three months after the closing and the third to be within six months after the closing. In addition, pursuant to a Securities Exchange Agreement, as amended on June 24, 2021 (the “Exchange Agreement”), by and among the Company, Sen Lang, SenlangBio and the Investor, dated June 13, 2021, the Investor shall have the right, exercisable between the six-month and five year-anniversaries of the respective initial closing and installment closings, to elect to exchange, from time to time, all or part of its then-owned equity ownership of SenlangBio for shares (the “Exchange Shares”) of Avalon Common Stock at a fixed exchange price of US$1.21 per share of Avalon Common Stock, which was the market price of the Avalon Common Stock as of the date of the Exchange Agreement under Nasdaq rules. In addition, the Exchange Agreement provides that the Investor may only exchange up to 10% of its total investment amount in any 30 day period.
The proxy statement attached to this letter and the enclosed Annual Report of Avalon to stockholders for the fiscal year ended December 31, 2020, which includes the information required by Rule 14a-3 of the Securities Exchange Act of 1934, provides you with information about the proposed Acquisition and the annual meeting. I encourage you to read the entire proxy statement and accompanying Annual Report carefully.
At the Avalon annual meeting of stockholders, Avalon will ask its stockholders to, among other things, elect the nine director nominees named in the proxy statement to hold office until the next annual meeting of stockholders and until their successors are duly elected and qualified, ratify the appointment of Marcum LLP as the Company’s independent auditors for the fiscal year ending December 31, 2021 and approve the issuance of the Acquisition Shares in connection with the Acquisition and the issuance of the Exchange Shares in connection with the Exchange Agreement in compliance with the rules of Nasdaq, each as described in the proxy statement.
Only stockholders of record at the close of business on [_], 2021, the record date for determining the stockholders entitled to notice of and to vote at the annual meeting, are entitled to notice of and to vote at the annual meeting and any adjournment thereof.
Your vote is very important. The Acquisition cannot be completed unless the issuances of the Acquisition Shares and the Exchange Shares are adopted by the affirmative vote of the holders of a majority of votes cast by the stockholders present in person or represented by proxy and entitled to vote thereon at the annual meeting.
Whether or not you are able to attend the annual meeting in person, please complete, sign and date the enclosed proxy card and return it in the envelope provided as soon as possible, or follow the instructions provided for submitting a proxy by telephone or the Internet. If you hold shares through a broker or other nominee, you should follow the procedures provided by your broker or other nominee. These actions will not limit your right to vote in person if you wish to attend the annual meeting and vote in person.
Thank you for your cooperation and your continued support of Avalon.
| Sincerely, | |
| Wenzhao “Daniel” Lu, | |
| Chairman of the Board of Directors |
This proxy statement is dated [_], 2021 and
is first being mailed along with the Annual Report to stockholders
on or about [_], 2021.
AVALON GLOBOCARE CORP.
4400 Route 9 South, Suite 3100
Freehold, New Jersey 07728
NOTICE OF ANNUAL MEETING OF STOCKHOLDERS
TO BE HELD ON [ ], 2021
To the Stockholders of Avalon GloboCare Corp.:
NOTICE IS HEREBY GIVEN that the annual meeting of stockholders (the “annual meeting”) of Avalon GloboCare Corp., a Delaware corporation (the “Company,” “Avalon,” “we,” “our” or “us”), will be held on [ ], 2021, at [ ] [a/p.m.], Eastern time at [ ]. Only stockholders who hold shares of Avalon common stock, $0.0001 par value per share (“Avalon Common Stock”) at the close of business on [ ], 2021, the record date for the annual meeting, are entitled to vote at the annual meeting and any adjournments or postponements thereof.
The annual meeting is being held for the following purposes:
| 1. | The “Director Election Proposal”—to elect the nine director nominees named in the proxy statement to hold office until the next annual meeting of stockholders and until their successors are duly elected and qualified; |
| 2. | The “Auditor Proposal”—to ratify the appointment of Marcum LLP as the Company’s independent auditors for the fiscal year ending December 31, 2021; and |
| 3. | The “Nasdaq Proposal”—to approve, pursuant to the rules of the Nasdaq Stock Market as described herein, the issuance of (i) 81 million shares (the “Acquisition Shares”) of the common stock, par value US$0.0001 per share, of Avalon (the “Avalon Common Stock”) issuable to the holders (the “Sen Lang Shareholders”) of the share capital of Lonlon Biotech Ltd., a company incorporated in the British Virgin Islands (“BVI”) (“Sen Lang”), pursuant to the terms of the Share Purchase Agreement, dated as of June 13, 2021 (as may be amended from time to time, the “Purchase Agreement”), by and among Avalon, Sen Lang, the ultimate beneficial owners of the Sen Lang Shareholders (the “Sen Lang Beneficial Shareholders” and, together with the Sen Lang Shareholders, the “Sen Lang Owners”) and a representative of the Sen Lang Owners (the “Sen Lang Representative”), pursuant to which Avalon will acquire (the “Acquisition”) all of the issued and outstanding share capital of Sen Lang (the “Sen Lang Shares”) and (ii) shares of Avalon Common Stock (the “Exchange Shares”) issuable upon the exchange of shares of Senlang Biotechnology Co. Ltd., a PRC domestic company with limited liability organized and existing under the laws of the PRC (the “OpCo” or “SenlangBio”) to be issued to an investor in a private placement of the equity of SenlangBio (all as described in more detail herein); | |
| 4. | The “Adjournment Proposal”—to approve the adjournment of the annual meeting to a later date or time, if necessary, to solicit additional proxies if, based upon the tabulated vote at the time of the annual meeting, there are not sufficient votes to approve the Nasdaq Proposal. |
After careful consideration, Avalon’s board of directors has unanimously determined that the forms, terms and provisions of the Purchase Agreement and the Exchange Agreement, including the Acquisition and the Equity Financing, are advisable and in the best interests of Avalon and its stockholders, and unanimously recommends you vote “FOR” Proposals 1 through 3, as well as the Adjournment Proposal.
Avalon will transact no other business at the annual meeting, except such business as may properly be brought before the annual meeting or any adjournment or postponement thereof. Please refer to the proxy statement of which this notice is a part for further information with respect to the business to be transacted at the annual meeting.
The approval of the Auditor Proposal, the Nasdaq Proposal, and the Adjournment Proposal requires the affirmative vote of the holders of a majority of votes cast by the stockholders present in person or represented by proxy and entitled to vote thereon at the annual meeting. The approval of the Director Election Proposal requires the affirmative vote of a plurality of the votes properly cast on the election of directors.
Completion of the Acquisition is conditioned upon, among other things, approval of the Nasdaq Proposal.
Your attention is directed to the proxy statement and Annual Report of Avalon to stockholders for the fiscal year ended December 31, 2020, which includes the information required by Rule 14a-3 of the Securities Exchange Act of 1934, accompanying this notice (including the financial statements and annexes attached thereto) for a more complete description of the proposed Acquisition and related transactions and each of our proposals. We encourage you to read this proxy statement and Annual Report carefully in its entirety. In particular, we urge you to read carefully the sections entitled “Risk Factors” beginning on page 18 of the accompanying proxy statement and in the Annual Report. If you have any questions or need assistance voting your shares, please call Avalon at 732-780-4400.
Your vote is very important, regardless of the number of shares of Avalon Common Stock that you own. Even if you plan to attend the annual meeting, we request that you complete, sign, date and return the enclosed proxy card in the envelope provided, or submit your proxy by telephone or the Internet prior to the annual meeting, and thus ensure that your shares will be represented and voted at the annual meeting if you later become unable to attend. If your shares are held in a stock brokerage account or by a bank or other nominee, please follow the instructions that you receive from your broker, bank or other nominee to vote your shares.
| By Order of the Board of Directors, | |
| /s/ Wenzhao “Daniel” Lu | |
| Wenzhao “Daniel” Lu | |
| [ ], 2021 | Chairman of the Board of Directors |
TABLE OF CONTENTS
i
ii
QUESTIONS AND ANSWERS ABOUT THE ANNUAL MEETING AND THE ACQUISITION
The following questions and answers briefly address some commonly asked questions regarding the proposed Acquisition and about the proposals to be presented at the Avalon annual meeting of stockholders, including with respect to the proposed Acquisition. The following questions and answers may not include all the information that is important to Avalon stockholders. Stockholders are urged to read carefully this entire proxy statement, including the financial statements and annexes attached hereto and the other documents referred to herein. Except where indicated, the information in this proxy statement does not give effect to the closing of the Equity Financing described in Proposal No. 3 of this proxy statement. References in this proxy statement to “$” refer to U.S. dollars.
| Q: | Why am I receiving this proxy statement? |
| A: | Avalon has furnished these materials to you by mail, in connection with the Company’s solicitation of proxies for use at the Annual Meeting of Stockholders to be held on [ ], 2021, at [ : ] [ ].m. Eastern Time. This year’s annual meeting of shareholders will be held as a virtual meeting. Stockholders attending the virtual meeting will be afforded the same rights and opportunities to participate as they would at an in-person meeting. You will be able to attend and participate in the annual meeting online via a live webcast by visiting [ ]. These materials have also been made available to you on the Internet. These materials describe the proposals on which the Company would like you to vote and also give you information on these proposals so that you can make an informed decision. We are furnishing our proxy materials on or about [ ], 2021 to all stockholders of record entitled to vote at the annual meeting. |
In addition, on June 13, 2021, Avalon GloboCare Corp., a Delaware corporation (the “Company” or “Avalon”), entered into a Share Purchase Agreement (the “Purchase Agreement”), by and among the Company, Lonlon Biotech Ltd., a company incorporated in the British Virgin Islands (“BVI”) (“Sen Lang”), the holders of the share capital of Sen Lang (the “Sen Lang Shareholders”), the ultimate beneficial owners of the Sen Lang Shareholders (the “Sen Lang Beneficial Shareholders” and, together with the Sen Lang Shareholders, the “Sen Lang Owners”) and a representative of the Sen Lang Owners (the “Sen Lang Representative”). Pursuant to the Purchase Agreement, subject to the satisfaction of the conditions to closing therein, including approval by the Avalon stockholders pursuant to the rules of the Nasdaq Stock Market (“Nasdaq”), Avalon agreed to purchase (the “Acquisition”) all of the issued and outstanding share capital of Sen Lang (the “Sen Lang Shares”).
Sen Lang, through a “variable interest entity” structure (described in more detail below under “VIE Structure”) of contractual rights held by its wholly-owned subsidiary Beijing Langlang Runfeng Biotechnology Co., Ltd., a wholly foreign owned enterprise with limited liability organized and existing under the laws of the People’s Republic of China (the “PRC”) (the “PRC Subsidiary”), has full economic benefit and management control over, and is consolidated for accounting purposes with, Senlang Biotechnology Co. Ltd., a PRC domestic company with limited liability organized and existing under the laws of the PRC (the “OpCo” or “SenlangBio”). SenlangBio is mainly engaged in the business of research and development in relation to CAR-T cell therapy, immune cell therapy and related drug development. SenlangBio is owned 100% by certain of the Sen Lang Beneficial Shareholders. A wholly-owned subsidiary of SenlangBio, Shijiazhuang Senlang Medical Laboratory Co., Ltd., a company with limited liability organized and existing under the laws of the PRC (“SenlangBio Clinical Laboratory”) is engaged in the business of testing of immunology, serology and molecular genetics specialties for patients, including hematology-tumor diagnostics and testing prior to clinical trials for cell therapy. For a more detailed discussion of the business of SenlangBio and SenlangBio Clinical Laboratory, please see the section entitled “Information about SenlangBio.”
Prior to the execution of the Purchase Agreement, the Board of Directors of Avalon (the “Board”), unanimously (i) determined that the terms and provisions of the Purchase Agreement and the transactions contemplated thereby, including the Acquisition, are fair to, advisable and in the best interests of the Company and its stockholders, (ii) approved the Purchase Agreement and the transactions contemplated thereby, including the Acquisition and the issuance of the Acquisition Shares (as defined below), (iii) authorized, empowered and directed the Company to perform all of its obligations under the Purchase Agreement and the Exchange Agreement (as defined below) and related documents, and (iv) resolved to recommend the approval by the stockholders of the Company of the issuance of the Acquisition Shares in connection with the Acquisition and the issuance of the Exchange Shares (as defined below) in connection with the Exchange Agreement in compliance with the rules of Nasdaq (the “Company Board Recommendation”).
1
The purchase price being paid by Avalon to the Sen Lang Shareholders under the Purchase Agreement for the Sen Lang Shares is an aggregate of 81 million shares (the “Acquisition Shares”) of the common stock, par value US$0.0001 per share, of Avalon (the “Avalon Common Stock”). Ten percent (10%), or 8.1 million, of such shares will be held in escrow for 12 months following the closing to satisfy any indemnification obligations of the Sen Lang Shareholders under the Share Purchase Agreement. In addition, at the closing of the Acquisition, it is expected that Dr. Jianqiang Li, scientific founder and CSO of SenlangBio, will join the board of the Company, and Dr. Li will also be appointed as Chief Technology Officer of the Company. The Acquisition Shares will not be registered under the Securities Act of 1933, as amended (the “Securities Act”) and, therefore, will be restricted securities under Rule 144 under the Securities Act for six months or longer after the closing of the Acquisition, subject to “affiliate” status with the Company under the Securities Act.
A copy of the Purchase Agreement is attached to this proxy statement as Annex A. For a more detailed discussion of the Purchase Agreement, please see the section entitled “The Purchase Agreement.”
In connection with the Acquisition, on June 13, 2021, an institutional investor (the “Investor”) entered into an agreement, as amended on June 24, 2021, with SenlangBio related to the purchase of registered capital of SenlangBio (the “OpCo Capital Increase Agreement”) pursuant to which the Investor will acquire an aggregate of up to 13.5% of the equity ownership of SenlangBio for an aggregate purchase price (the “Subscription Amount) of approximately US$30,000,000 (represented by an actual investment of RMB200,000,000) (the “Equity Financing”), which funds will be invested in SenlangBio in three equal installments of approximately US$10,000,000, at a fixed price, the first to be upon the closing of the Acquisition, the second to be within three months after the closing and the third to be within six months after the closing. In addition, pursuant to a Securities Exchange Agreement, as amended on June 24, 2021 (the “Exchange Agreement”), by and among the Company, Sen Lang, SenlangBio and the Investor, dated June 13, 2021, the Investor shall have the right, exercisable between the six-month and five year-anniversaries of the respective initial closing and installment closings, to elect to exchange, from time to time, all or part of its then-owned equity ownership of SenlangBio for shares (the “Exchange Shares”) of Avalon Common Stock at a fixed exchange price of US$1.21 per share of Avalon Common Stock, which was the market price of the Avalon Common Stock as of the date of the Exchange Agreement under Nasdaq rules. In addition, the Exchange Agreement provides that the Investor may only exchange up to 10% of its total investment amount in any 30 day period.
This proxy statement and its annexes contain important information about the proposed Acquisition and Equity Financing and the proposals to be acted upon at the annual meeting. You should read this proxy statement and its annexes and the Annual Report, which is incorporated in its entirety into this proxy statement by reference, carefully and in their entirety.
Q: What is included in these materials?
A: These materials include:
| ● | this Proxy Statement for the annual meeting; and |
| ● | the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2020 (the “Annual Report”). |
Q: What is the proxy card?
A: The proxy card enables you to appoint David Jin, our Chief Executive Officer, and Luisa Ingargiola, our Chief Financial Officer, as your representatives at the annual meeting. By completing and returning a proxy card, you are authorizing these individuals to vote your shares at the annual meeting in accordance with your instructions on the proxy card. This way, your shares will be voted whether or not you attend the annual meeting.
2
| Q: | What matters will stockholders consider at the Avalon annual meeting? |
| A: | At the Avalon annual meeting of stockholders, Avalon will ask its stockholders to vote in favor of the following proposals (the “Avalon Proposals”): |
| ● | Proposal 1—to elect the nine director nominees named in the proxy statement to hold office until the next annual meeting of stockholders and until their successors are duly elected and qualified (the “Director Election Proposal”); |
| ● | Proposal 2—to ratify the appointment of Marcum LLP as the Company’s independent auditors for the fiscal year ending December 31, 2021 (the “Auditor Proposal”); and |
| ● | Proposal 3—to approve, pursuant to the rules of the Nasdaq Stock Market, the issuance of (i) the Acquisition Shares pursuant to the terms of the Purchase Agreement and (ii) the Exchange Shares (the “Nasdaq Proposal”); |
| ● | Proposal 4—to adjourn the annual meeting, if necessary, to another time or place to solicit additional proxies if there are not sufficient votes in favor of Proposal 1 (the “Adjournment Proposal”). |
| Q: | What will happen upon the consummation of the Acquisition and the Equity Financing? |
| A: | On the date of closing of the Acquisition (the “Closing Date”), Avalon will issue the Acquisition Shares to the Sen Lang Shareholders in exchange for all of the outstanding equity of Sen Lang, and will thereby acquire the full economic benefit and management control over, and consolidate for accounting purposes with, SenlangBio. In addition, the Equity Financing will be consummated, whereby the Investor will begin to acquire up to approximately 13.5% of the equity ownership of SenlangBio for an aggregate purchase price of approximately US$30 million (represented by an actual investment of RMB200,000,000), which funds will be invested in SenlangBio in three equal installments of approximately US$10,000,000, at a fixed price, the first to be on the Closing Date, the second to be within three months after the closing and the third to be within six months after the closing. |
| Q: | Why is Avalon proposing to effect the Acquisition? |
| A: | Avalon believes that the post-Acquisition company will have several potential advantages, including: (i) a diverse pipeline of cell therapy product candidates, (ii) expanded footprint in China, (iii) operational synergies and (iv) an experienced management team. |
For a more complete discussion of Avalon’s reasons for the Acquisition, please see the sections entitled “The Acquisition—Reasons for the Acquisition.”
| Q: | What equity stake will current Avalon stockholders and the Sen Lang Shareholders have in Avalon after the closing of the Acquisition and prior to the consummation of the Equity Financing? |
| A: | It is anticipated that, upon the consummation of the Acquisition, the ownership of Avalon will be as follows: |
| ● | Current Avalon stockholders will own 51.0% of the total voting shares outstanding; and |
| ● | Current Sen Lang Shareholders will own 49.0% of the total voting shares outstanding. |
The numbers of shares and percentage interests set forth above do not take into account (i) shares of Avalon Common Stock issuable upon the exchange of shares of SenlangBio purchased by the Investor in the Equity Financing, pursuant to the Exchange Agreement, (ii) shares of Avalon Common Stock issuable upon the exercise of outstanding options and warrants and (iii) potential future issuances of Avalon securities.
In addition, under the Exchange Agreement, the Investor shall have the right, exercisable between the six-month and five year-anniversaries of the respective initial closing and installment closings, to elect to exchange, from time to time, all or part of its equity ownership of SenlangBio for Exchange Shares of Avalon at an effective exchange price of $1.21 per share of Avalon Common Stock. Following the completion of the financing and assuming the full funding by the investor in the financing, the aggregate number of shares of Avalon Common Stock that would be issuable under the Exchange Agreement (assuming the exchange of all shares) would be approximately 25,885,000 (using the conversion rate of US dollars to RMB of 6.3856 as of June 11, 2021). The resultant equity ownership of Avalon would be as follows:
| ● | Current Avalon stockholders will own 44.1% of the total voting shares outstanding; |
| ● | Current Sen Lang Shareholders will own 42.4% of the total voting shares outstanding and |
| ● | The Investor will own 13.5% of the total voting shares outstanding. |
3
| Q: | Who will be the officers and directors of the post-Acquisition company if the Acquisition is consummated? |
| A: | It is currently expected that Dr. Jianqiang Li, scientific founder and CSO of SenlangBio, will join the board of Avalon, and Dr. Li will also be appointed as Chief Technology Officer of Avalon. Meng Li, Avalon’s Chief Operating Officer and a director, will resign from her position on the board of Avalon. The Avalon board and management will otherwise remain the same aside from Dr. Li, with Wenzhao Lu (Chairman), David Jin, MD, PhD, Steven A. Sanders, Yancen Lu, Wilbert J. Tauzin II, Willliam B. Stilley, III, Tevi Troy and Yue “Charles” Li continuing to serve on the board and Dr. Jin continuing to serve as President and Chief Executive Officer, Ms. Li as Chief Operating Officer and Luisa Ingargiola as Chief Financial Officer. |
Please see the section entitled “Management After the Acquisition.”
| Q: | What is the VIE Structure? |
| A: | As a part of the restructure of Sen Lang, its subsidiaries and SenlangBio and its subsidiary (collectively, the “Acquired Companies”) before the closing of the Acquisition, SenlangBio and SenlangBio Clinical Laboratory will be controlled by the PRC Subsidiary by entering into a series of variable interest entities agreements among the PRC Subsidiary, SenlangBio and all current shareholders of SenlangBio, as well as the Investor (such agreements are collectively referred to as the “VIE Agreements”, and such contractual control arrangement is referred to as the “VIE Structure”). |
In the PRC, the VIE structure has become a popular and widely used overseas listing mode for enterprises in the sectors which are foreign investment restricted or prohibited, like the development and application of genetic diagnosis and treatment technology, which includes SenlangBio’s business. The VIE structure refers to an agreement mode in which the PRC domestic operating entity is separated from the overseas-listed entity, and the overseas-listed party/holding company controls the domestic operating entity by signing relevant agreements with the parties who would otherwise receive the benefits of ownership of SenlangBio and control its operations (i.e., VIE Agreements), and is able to consolidate the financial statements of the PRC domestic operating entity into the overseas-listed entity/holding company. After the completion of the overall VIE Structure, the interests/profits from the domestic operation, as well as operational control, have been transferred to the overseas listing/holding company.
It is a condition to closing under the Purchase Agreement that SenlangBio, the PRC Subsidiary and the shareholders of SenlangBio (including the Investor) execute the VIE Agreements. These VIE Agreements include (i) an exclusive technical consultation and service agreement; (ii) an exclusive purchase option agreement; (iii) an entrustment agreement of shareholders’ rights; (iv) a share pledge agreement; and (v) a spouse consent letter.
| Q: | What conditions must be satisfied to complete the Acquisition? |
| A: | There are a number of closing conditions in the Purchase Agreement, including that Avalon’s stockholders approve the issuance of the Acquisition Shares and the Exchange Shares in accordance with Nasdaq rules and that Avalon consummate the Equity Financing. In addition, the VIE Agreements (as defined below) must have been executed and delivered. For a summary of the conditions that must be satisfied or waived prior to completion of the Acquisition, please see the section entitled “The Purchase Agreement—Conditions to the Closing of the Acquisition.” |
| Q: | What vote is required to approve the proposals presented at the Avalon annual meeting of stockholders? |
| A: | The approval of each of the Auditor Proposal, the Nasdaq Proposal, and the Adjournment Proposal requires the affirmative vote of the holders of a majority of votes cast by the stockholders present in person or represented by proxy and entitled to vote thereon at the annual meeting, and the approval of the Director Election Proposal requires the affirmative vote of a plurality of the votes properly cast on the election of directors. Accordingly, abstentions and broker non-votes, if any, will have no effect on the outcome of the Director Election Proposal, the Auditor Proposal, the Nasdaq Proposal and the Adjournment Proposal. |
| Q: | How much stock is owned by 5% stockholders, directors, and executive officers? |
| A: | As of June 30, 2021, Avalon’s directors and executive officers beneficially owned approximately 66.3% of the shares of Avalon Common Stock (calculated in accordance with SEC rules that define beneficial ownership) and owned 54,145,161 shares, 63.6% of the issued and outstanding Avalon Common Stock on such date. Although under no contractual or other obligation to do so, all of such directors and executive officers are currently expected to vote in favor of all of the Avalon Proposals, including the Nasdaq Proposal. |
4
| Q: | Are there any subsequent approvals required from the Acquired Companies’ shareholders to approve the Acquisition? |
| A: | No. All of the Sen Lang Shareholders and Sen Lang Beneficial Shareholders executed the Purchase Agreement, and all of the equity of SenlangBio is owned by certain of the Sen Lang Beneficial Shareholders. Therefore, no other proceedings are necessary for the consummation of the Acquisition, other than the execution and delivery of the VIE Agreements by the Investor and the Sen Lang Beneficial Shareholders who are the owners of SenlangBio. |
| Q: | How many votes do Avalon stockholders have at the annual meeting of stockholders? |
| A: | Each share of Avalon Common Stock is entitled to one vote at the annual meeting for each share of Avalon Common Stock held of record as of the record date. As of the close of business on the record date, there were [ ] outstanding shares of Avalon Common Stock. |
| Q: | What interests do Avalon’s current officers and directors have in the Acquisition? |
| A: | Avalon’s board of directors and executive officers may have interests in the Acquisition that are different from, in addition to or in conflict with, yours. |
As of June 30, 2021, Avalon’s directors and executive officers beneficially owned approximately 66.3% of the shares of Avalon Common Stock (calculated in accordance with SEC rules that define beneficial ownership). All of the current executive officers of Avalon will continue in their current positions after the Acquisition, and all of the directors except for Meng Li will continue on the Avalon board after the Acquisition. In addition, On April 10, 2020, in the ordinary course of business, SenlangBio entered into a scientific research project cooperation agreement with Beijing Lu Daopei Hospital Co., Ltd., under which Beijing Lu Daopei Hospital Co., Ltd. conducts scientific research for the interest of SenlangBio on the cytoplasmic CD79a antibody gated multicolor flow cytometry monitoring CD19-CAR-T bridging allogeneic transplantation for the treatment of refractory and relapsed acute B lymphocytic leukemia. SenlangBio provides research funds in the amount of RMB 2 million to Beijing Lu Daopei Hospital Co., Ltd. Beijing Lu Daopei Hospital Co., Ltd. is a wholly-owned subsidiary of an entity whose chairman is Wenzhao Lu, the Chairman and largest shareholder of Avalon.
For more information, please see the sections entitled “The Acquisition—Interests of Avalon’s Directors and Officers in the Acquisition.”
| Q: | What are the U.S. federal income tax consequences of the Acquisition? |
| A: | Neither Avalon nor its stockholders are expected to recognize federal income tax or gain as a result of the Acquisition. |
| Q: | Do Avalon stockholders have appraisal rights if they object to the proposed Acquisition? |
| A: | No. There are no appraisal rights available to holders of shares of Avalon Common Stock in connection with the Acquisition. |
| Q: | What will happen to Avalon if, for any reason, the Acquisition does not close? |
| A: | There are certain circumstances under which the Purchase Agreement may be terminated. Please see the section entitled “The Purchase Agreement—Termination of the Purchase Agreement” for information regarding the parties’ specific termination rights. |
If, as a result of the termination of the Purchase Agreement or otherwise, Avalon is unable to complete the Acquisition by December 31, 2021 or obtain the approval to extend the deadline for Avalon to consummate the Acquisition, the Avalon board of directors may elect to, among other things, attempt to complete another strategic transaction like the Acquisition, attempt to purchase additional assets, enter into collaboration or joint venture agreements or attempt to sell or otherwise dispose of the various assets of Avalon.
| Q: | When is the Acquisition expected to be completed? |
| A: | It is currently anticipated that the Acquisition will be consummated promptly following the Avalon annual meeting of stockholders, provided that all other conditions to the consummation of the Acquisition have been satisfied or waived, including consummation of the Equity Financing. For a description of the conditions to the completion of the Acquisition, please see the section entitled “The Purchase Agreement—Conditions to the Closing of the Acquisition.” |
5
| Q: | When and where will the annual meeting of Avalon stockholders be held? |
| A: | The Avalon annual meeting will be held in a virtual meeting format only. The annual meeting will be held on [ ], 2021 at [ ] [a/p].m. Eastern time. In order to participate in the annual meeting live via the Internet, you must register at [ ] by 11:59 p.m. Eastern Time by [ ], 2021. On the day of the Avalon annual meeting, if you have properly registered, you may enter the annual meeting by logging in using the event password you received via email in your registration confirmation at [ ]. You will not be able to attend the Avalon annual meeting in-person. |
If you are a registered holder, you must register using the virtual control number included in your proxy materials or your proxy card. If you hold your shares beneficially through a bank or broker, you must provide a legal proxy from your bank or broker during registration and you will be assigned a virtual control number in order to vote your shares during the Annual meeting. If you are unable to obtain a legal proxy to vote your shares, you will still be able to attend the Annual meeting (but will not be able to vote your shares) so long as you demonstrate proof of stock ownership. Instructions on how to connect and participate via the Internet, including how to demonstrate proof of stock ownership, are posted at [ ].
| Q: | What do I need to do now? |
| A | You are urged to carefully read and consider the information contained in this proxy statement, including the financial statements and annexes attached hereto, and in the Annual Report and to consider how the Acquisition will affect you. You should then vote as soon as possible in accordance with the instructions provided in this proxy statement on the enclosed proxy card or, if you hold your shares through a brokerage firm, bank or other nominee, on the voting instruction form provided by the broker, bank or nominee. |
| Q: | How do I vote? |
| A: | If you were a holder of Avalon Common Stock on [ ], 2021, the record date for the annual meeting of stockholders, you may provide your proxy instructions in one of three different ways. First, you can mail your signed proxy card in the enclosed return envelope. Second, you may provide your proxy instructions via the Internet by following the instructions on your proxy card or voting instruction form. You may also vote your shares at the annual meeting via live webcast. Please provide your proxy instructions only once, unless you are revoking a previously delivered proxy instruction, and as soon as possible so that your shares can be voted at the annual meeting of Avalon stockholders. |
| Q: | What happens if I do not return a proxy card or otherwise provide proxy instructions, as applicable? |
| A: | Signed and dated proxies received by Avalon without an indication of how the stockholder intends to vote on a proposal will be voted “FOR” each of the Avalon Proposals presented to Avalon’s stockholders in accordance with the recommendation of Avalon’s board of directors. The proxyholders may use their discretion to vote on any other matters which properly come before the Avalon annual meeting. |
| Q: | May I vote in person at the annual meeting of stockholders of Avalon? |
| A: | Due to the public health impact of the coronavirus outbreak (“COVID-19”) and to support the health and well-being of Avalon’s stockholders, the Avalon annual meeting will be held in a virtual meeting format only. If your shares of Avalon Common Stock are registered directly in your name with the Avalon transfer agent, you are considered to be the stockholder of record with respect to those shares, and the proxy materials and proxy card are being sent directly to you by Avalon. If you are an Avalon stockholder of record, you must register using the virtual control number included in your proxy materials or your proxy card (if you received a printed copy of the proxy materials) in order to vote at the annual meeting. If you hold your shares beneficially through a bank or broker, you are considered the beneficial owner of shares held in “street name” and you must provide a legal proxy from your bank or broker during registration and you will be assigned a virtual control number in order to vote your shares during the annual meeting. If you are unable to obtain a legal proxy to vote your shares, you will still be able to attend the annual meeting, so long as you demonstrate proof of stock ownership, but you will not be able to vote your shares. Even if you plan to attend the Avalon annual meeting live via the internet, Avalon encourages you to vote in advance by internet or mail so that your vote will be counted if you later decide not to attend the annual meeting live via the internet. |
For more information, please see the section entitled “The Annual Meeting of Avalon Stockholders—Voting Your Shares.”
6
| Q: | If my Avalon shares are held in “street name” by my broker, will my broker vote my shares for me? |
| A: | If you are a beneficial owner of shares of Avalon Common Stock and do not instruct your broker, bank, or other agent how to vote your shares, the question of whether your broker or nominee will still be able to vote your shares depends on whether the New York Stock Exchange (the “NYSE”), deems the particular proposal to be a “routine” matter and how your broker or nominee exercises any discretion they may have in the voting of the shares that you beneficially own. Brokers and nominees can use their discretion to vote “uninstructed” shares with respect to matters that are considered to be “routine,” but not with respect to “non-routine” matters. Under the rules and interpretations of the NYSE, “non-routine” matters are matters that may substantially affect the rights or privileges of stockholder, such as mergers, stockholder proposals, elections of directors (even if not contested), executive compensation (including any advisory stockholder votes on executive compensation and on the frequency of stockholder votes on executive compensation), and certain corporate governance proposals, even if management-supported. |
For any Avalon Proposal that is considered a “routine” matter, your broker or nominee may vote your shares in its discretion either for or against the proposal even in the absence of your instruction. For any Avalon Proposal that is considered a “non-routine” matter for which you do not give your broker instructions, the shares will be treated as broker non-votes. Broker non-votes occur when a beneficial owner of shares held in street name does not give instructions to the broker or nominee holding the shares as to how to vote on matters deemed “non-routine.” Broker non-votes will not be considered to be shares “entitled to vote” at the annual meeting and will not be counted as having been voted on the applicable proposal. Avalon currently anticipates that only the Auditor Proposal is likely to be deemed routine by the NYSE.
| Q: | May I change my vote after I have submitted a proxy or provided proxy instructions? |
| A: | Yes. Avalon stockholders of record may change their vote at any time before their proxy is voted at the Avalon annual meeting, as applicable, in one of the following ways: |
| ● | filing with the Secretary of Avalon, a letter revoking the proxy; |
| ● | submitting another signed proxy with a later date; or |
| ● | attending the Avalon annual meeting and voting online, provided you file a written revocation with the Secretary of the Avalon annual meeting prior to the voting of such proxy. |
| Q: | What is the quorum requirement for the annual meeting of stockholders? |
| A: | The holders of at least a majority of the voting power of the outstanding shares of Avalon Common Stock entitled to vote, as of the record date, represented in person or by proxy, will constitute a quorum for the transaction of business at the Avalon annual meeting. Proxies marked as abstentions and broker non-votes, if any, will be included to determine the number of shares present at the annual meeting. In the absence of a quorum, a majority of Avalon’s stockholders, present in person or represented by proxy, and voting thereon will have the power to adjourn the annual meeting. As of the record date for the annual meeting, [ ] shares of Avalon Common Stock would be required to achieve a quorum. |
7
| Q: | What risks should I consider in deciding whether to vote in favor of the Avalon Proposals? |
| A: | You should carefully review this proxy statement and the Annual Report, including the sections entitled “Risk Factors,” which set forth certain risks and uncertainties related to the Acquisition, risks and uncertainties to which the post-Acquisition company’s business will be subject, and risks and uncertainties to which each of Avalon and SenlangBio, as an independent company, is subject. |
Q: How are proxy materials delivered to households?
A: Only one copy of the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2020 and this Proxy Statement will be delivered to an address where two or more stockholders reside with the same last name or who otherwise reasonably appear to be members of the same family based on the stockholders’ prior express or implied consent.
We will deliver promptly upon written or oral request a separate copy of the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2020 and this Proxy Statement. If you share an address with at least one other stockholder, currently receive one copy of our Annual Report on Form 10-K and Proxy Statement at your residence, and would like to receive a separate copy of our Annual Report on Form 10-K and Proxy Statement for future stockholder meetings of the Company, please specify such request in writing and send such written request to Avalon GloboCare Corp., 4400 Route 9 South, Suite 3100, Freehold, New Jersey 07728; Attention: Chief Financial Officer.
If you want to receive separate copies of the Company’s proxy statement and annual report in the future, or if you are receiving multiple copies and would like to receive only one copy for your household, you should contact your bank, broker or other nominee record holder, or you may contact us at Avalon’s address and telephone number.
| Q: | Who will solicit and pay the cost of soliciting proxies? |
| A: | Avalon will bear all costs and expenses in connection with the solicitation of proxies, including the costs of preparing, printing and mailing this proxy statement and the Annual Report for the Avalon annual meeting. |
| Q: | Who can help answer my questions? |
| A: | If you are an Avalon stockholder and would like additional copies, without charge, of this proxy statement and the Annual Report, or if you have questions about the Acquisition, including the procedures for voting your shares, you should contact Avalon at: |
Avalon GloboCare Corp.
4400 Route 9 South, Suite 3100
Freehold, New Jersey 07728
732-780-4400
8
SUMMARY OF THE PROXY STATEMENT
This summary highlights selected information from this proxy statement and does not contain all of the information that is important to you. To better understand the Acquisition and the other proposals to be considered at the annual meeting, you should read this entire proxy statement carefully, including the annexes. Please see the section entitled “Where You Can Find More Information.”
The Annual Meeting
The annual meeting is being held for the following purposes:
| 1. | The “Director Election Proposal”—to elect the nine director nominees named in the proxy statement to hold office until the next annual meeting of stockholders and until their successors are duly elected and qualified; |
| 2. | The “Auditor Proposal”—to ratify the appointment of Marcum LLP as the Company’s independent auditors for the fiscal year ending December 31, 2021; and |
| 3. | The “Nasdaq Proposal”— to approve, pursuant to the rules of the Nasdaq Stock Market, the issuance of (i) the Acquisition Shares pursuant to the terms of the Purchase Agreement and (ii) the Exchange Shares (the “Nasdaq Proposal”); | |
| 4. | The “Adjournment Proposal”—to approve the adjournment of the annual meeting to a later date or time, if necessary, to solicit additional proxies if, based upon the tabulated vote at the time of the annual meeting, there are not sufficient votes to approve the Nasdaq Proposal. |
Avalon
Avalon GloboCare Corp. is a clinical-stage, vertically integrated, leading CellTech bio-developer dedicated to advancing and empowering innovative, transformative immune effector cell therapy, exosome technology, as well as COVID-19 related diagnostics and therapeutics. Avalon also provides strategic advisory and outsourcing services to facilitate and enhance its clients' growth and development, as well as competitiveness in healthcare and CellTech industry markets. Through its subsidiary structure with unique integration of verticals from innovative R&D to automated bioproduction and accelerated clinical development, Avalon is establishing a leading role in the fields of cellular immunotherapy (including CAR-T/NK), exosome technology (ACTEX™), and regenerative therapeutics.
The principal executive offices of Avalon are located at 4400 Route 9 South, Suite 3100, Freehold, New Jersey 07728, and its telephone number is (732) 780-4400.
SenlangBio
SenlangBio is a clinical-stage biotechnology company that focuses on three advanced technology platforms—CAR T-cells, CAR γδT-cells and armTILs—to develop a robust pipeline of innovative and transformative cellular immunotherapies for cancer patients. Chimeric antigen receptor (CAR) T-cells (CAR-T) are natural cell-killing T-cells that have been engineered to specifically recognize and kill cancerous cells. Allogeneic (universal) CAR γδT-cells (CAR-GDT) are a specific class of donor-derived T-cells that can provide superior anti-tumor effectiveness. Armored tumor infiltrating lymphocytes (armTILs) are cancer-killing T-cells that provide a unique “personalized” cellular immunotherapy approach.
SenlangBio is currently the largest cell therapy company in Northern China in terms of the scale of bio-manufacturing, as well as the breadth and depth of active pre-clinical research and clinical development programs.
A wholly-owned subsidiary of SenlangBio, Shijiazhuang Senlang Medical Laboratory Co., Ltd., a company with limited liability organized and existing under the laws of the PRC (“SenlangBio Clinical Laboratory”) is engaged in the business of testing of immunology, serology and molecular genetics specialties for patients, including hematology-tumor diagnostics and testing prior to clinical trials for cell therapy.
The principal executive offices of SenlangBio are located at Room 512 and 513, Building 1, 136 Yellow River Avenue, Shijiazhuang High-tech Development Zone, Hebei Province, China, and its telephone number is +86-311-82970975.
9
Sen Lang
Lonlon Biotech Ltd., a company incorporated in the British Virgin Islands (“BVI”) (“Sen Lang”), the ultimate beneficial owner and controlling entity of SenlangBio through the VIE Structure, was established on October 15, 2020 to be a holding company of Lonlon Biotech Investment Limited (“Senlang HK”), which owns 100% of the equity of Beijing Langlang Runfeng Biotechnology Co., Ltd., a wholly foreign owned enterprise with limited liability organized and existing under the laws of the PRC (the “PRC Subsidiary”), and the PRC Subsidiary is the beneficiary of SenlangBio through the VIE Structure.
The principal executive offices of Sen Lang are located at 5F, Huabin Center, Jianguomen Wai Ave, Chaoyang District, Beijing, China, 100022.
On June 13, 2021, Avalon GloboCare Corp., a Delaware corporation (the “Company” or “Avalon”), entered into a Share Purchase Agreement (the “Purchase Agreement”), by and among the Company, Lonlon Biotech Ltd., a company incorporated in the British Virgin Islands (“BVI”) (“Sen Lang”), the holders of the share capital of Sen Lang (the “Sen Lang Shareholders”), the ultimate beneficial owners of the Sen Lang Shareholders (the “Sen Lang Beneficial Shareholders” and, together with the Sen Lang Shareholders, the “Sen Lang Owners”) and a representative of the Sen Lang Owners (the “Sen Lang Representative”). Pursuant to the Purchase Agreement, subject to the satisfaction of the conditions to closing therein, including approval by the Avalon stockholders pursuant to the rules of the Nasdaq Stock Market (“Nasdaq”), Avalon agreed to purchase (the “Acquisition”) all of the issued and outstanding share capital of Sen Lang (the “Sen Lang Shares”).
Sen Lang, through a “variable interest entity” structure (described in more detail below under “VIE Structure”) of contractual rights held by its wholly-owned subsidiary Beijing Langlang Runfeng Biotechnology Co., Ltd., a wholly foreign owned enterprise with limited liability organized and existing under the laws of the People’s Republic of China (the “PRC”) (the “PRC Subsidiary”), has full economic benefit and management control over, and is consolidated for accounting purposes with, Senlang Biotechnology Co. Ltd., a PRC domestic company with limited liability organized and existing under the laws of the PRC (the “OpCo” or “SenlangBio”). SenlangBio is mainly engaged in the business of research and development in relation to CAR-T cell therapy, immune cell therapy and related drug development. SenlangBio is owned 100% by certain of the Sen Lang Beneficial Shareholders. A wholly-owned subsidiary of SenlangBio, Shijiazhuang Senlang Medical Laboratory Co., Ltd., a company with limited liability organized and existing under the laws of the PRC (“SenlangBio Clinical Laboratory”) is engaged in the business of testing of immunology, serology and molecular genetics specialties for patients, including hematology-tumor diagnostics and testing prior to clinical trials for cell therapy. For a more detailed discussion of the business of SenlangBio and SenlangBio Clinical Laboratory, please see the section entitled “Information about SenlangBio.”
The purchase price being paid by Avalon to the Sen Lang Shareholders under the Purchase Agreement for the Sen Lang Shares is an aggregate of 81 million shares (the “Acquisition Shares”) of the common stock, par value US$0.0001 per share, of Avalon (the “Avalon Common Stock”). Ten percent (10%), or 8.1 million, of such shares will be held in escrow for 12 months following the closing to satisfy any indemnification obligations of the Sen Lang Shareholders under the Share Purchase Agreement. In addition, at the closing of the Acquisition, it is expected that Dr. Jianqiang Li, scientific founder and CSO of SenlangBio, will join the board of the Company, and Dr. Li will also be appointed as Chief Technology Officer of the Company. The Acquisition Shares will not be registered under the Securities Act of 1933, as amended (the “Securities Act”) and, therefore, will be restricted securities under Rule 144 under the Securities Act for six months or longer after the closing of the Acquisition, subject to “affiliate” status with the Company under the Securities Act.
A copy of the Purchase Agreement is attached to this proxy statement as Annex A. For a more detailed discussion of the Purchase Agreement, please see the section entitled “The Purchase Agreement.”
10
Conditions to the Closing of the Acquisition
The Avalon stockholders must approve the Nasdaq Proposal. The approval of the Nasdaq Proposal requires the affirmative vote of the holders of a majority of votes cast by the stockholders present in person or represented by proxy and entitled to vote thereon at the annual meeting.
In addition, Avalon must consummate the Equity Financing and the VIE Agreements must have been executed and delivered. Please see the section entitled “The Purchase Agreement—Conditions to the Closing of the Acquisition.”
Non-Solicitation
In the Purchase Agreement, Sen Lang and its affiliates agreed not to (i) solicit, initiate, or encourage the submission of any proposal or offer from any person relating to the acquisition of the equity or assets of Sen Lang, its subsidiaries and SenlangBio and its subsidiaries (collectively, the “Acquired Companies”) or (ii) enter into or renew any distribution agreement related to the business of the Acquired Companies, in each case without the prior written consent of Avalon.
Termination of the Purchase Agreement
Either Avalon or the Sen Lang Representative can terminate the Purchase Agreement under certain circumstances, which would prevent the Acquisition from being consummated. For more information, please see the section entitled “The Purchase Agreement—Termination of the Purchase Agreement.”
In connection with the Acquisition, on June 13, 2021, an institutional investor (the “Investor”) entered into an agreement, as amended on June 24, 2021, with SenlangBio related to the purchase of registered capital of SenlangBio (the “OpCo Capital Increase Agreement”) pursuant to which the Investor will acquire an aggregate of up to 13.5% of the equity ownership of SenlangBio for an aggregate purchase price (the “Subscription Amount) of approximately US$30,000,000 (represented by an actual investment of RMB200,000,000) (the “Equity Financing”), which funds will be invested in SenlangBio in three equal installments of approximately US$10,000,000, at a fixed price, the first to be upon the closing of the Acquisition, the second to be within three months after the closing and the third to be within six months after the closing. In addition, pursuant to a Securities Exchange Agreement, as amended on June 24, 2021 (the “Exchange Agreement”), by and among the Company, Sen Lang, SenlangBio and the Investor, dated June 13, 2021, the Investor shall have the right, exercisable between the six-month and five year-anniversaries of the respective initial closing and installment closings, to elect to exchange, from time to time, all or part of its then-owned equity ownership of SenlangBio for shares (the “Exchange Shares”) of Avalon Common Stock at a fixed exchange price of US$1.21 per share of Avalon Common Stock, which was the market price of the Avalon Common Stock as of the date of the Exchange Agreement under Nasdaq rules. In addition, the Exchange Agreement provides that the Investor may only exchange up to 10% of its total investment amount in any 30 day period.
As a part of the restructure of Sen Lang, its subsidiaries and SenlangBio and its subsidiaries (collectively, the “Acquired Companies”) before the closing of the Acquisition, SenlangBio and SenlangBio Clinical Laboratory will be controlled by the PRC Subsidiary by entering into a series of variable interest entities agreements among the PRC Subsidiary, SenlangBio and all current shareholders of SenlangBio, as well as the Investor (such agreements are collectively referred to as the “VIE Agreements”, and such contractual control arrangement is referred to as the “VIE Structure”).
11
In the PRC, the VIE structure has become a popular and widely used overseas listing mode for enterprises in the sectors which are foreign investment restricted or prohibited, like the development and application of genetic diagnosis and treatment technology, which includes SenlangBio’s business. The VIE structure refers to an agreement mode in which the PRC domestic operating entity is separated from the overseas-listed entity, and the overseas-listed party/holding company controls the domestic operating entity by signing relevant agreements with the parties who would otherwise receive the benefits of ownership of SenlangBio and control its operations (i.e., VIE Agreements), and is able to consolidate the financial statements of the PRC domestic operating entity into the overseas-listed entity/holding company. After the completion of the overall VIE Structure, the interests/profits from the domestic operation, as well as operational control, have been transferred to the overseas listing/holding company.
It is a condition to closing under the Purchase Agreement that SenlangBio, the PRC Subsidiary and the shareholders of SenlangBio (including the Investor) execute the VIE Agreements. These VIE Agreements include (i) an exclusive technical consultation and service agreement; (ii) an exclusive purchase option agreement; (iii) an entrustment agreement of shareholders’ rights; (iv) a share pledge agreement; and (v) a spouse consent letter.
Interests of Certain Persons in the Acquisition
Avalon
In considering the recommendation of Avalon’s board of directors to vote in favor of the Avalon Proposals, stockholders should be aware that, aside from their interests as stockholders, certain Avalon directors and officers have interests in the Acquisition that are different from, or in addition to, those of other stockholders generally. Avalon’s directors were aware of and considered these interests, among other matters, in evaluating the Acquisition, and in recommending to stockholders that they approve the Avalon Proposals. Stockholders should take these interests into account in deciding whether to approve the Avalon Proposals.
As of June 30, 2021, Avalon’s directors and executive officers beneficially owned approximately 66.3% of the shares of Avalon Common Stock (calculated in accordance with SEC rules that define beneficial ownership). All of the current executive officers of Avalon will continue in their current positions after the Acquisition, and all of the directors except for Meng Li will continue on the Avalon board after the Acquisition. In addition, on April 10, 2020, in the ordinary course of business, SenlangBio entered into a scientific research project cooperation agreement with Beijing Lu Daopei Hospital Co., Ltd., under which Beijing Lu Daopei Hospital Co., Ltd. conducts scientific research for the interest of SenlangBio on the cytoplasmic CD79a antibody gated multicolor flow cytometry monitoring CD19-CAR-T bridging allogeneic transplantation for the treatment of refractory and relapsed acute B lymphocytic leukemia. SenlangBio provides research funds in the amount of RMB 2 million to Beijing Lu Daopei Hospital Co., Ltd. Beijing Lu Daopei Hospital Co., Ltd. is a wholly-owned subsidiary of an entity whose chairman is Wenzhao Lu, the Chairman and largest shareholder of Avalon.
These interests may influence Avalon’s board of directors in making their recommendation that you vote in favor of the approval of the Avalon Proposals.
For more information, please see the section entitled “The Acquisition—Interests of Avalon’s Directors and Officers in the Acquisition.”
Reasons for the Approval of the Acquisition
After careful consideration, Avalon’s board of directors recommends that Avalon stockholders vote “FOR” each of the Avalon Proposals being submitted to a vote of the Avalon stockholders at the Avalon annual meeting of stockholders.
For a description of Avalon’s reasons for the approval of the Acquisition and the recommendation of its board of directors, see the section entitled “The Acquisition—Reasons for the Acquisition.”
12
Regulatory Approvals Required for the Acquisition
Avalon must comply with applicable federal and state securities laws and the rules and regulations of Nasdaq in connection with the issuance of shares of Avalon Common Stock in connection with the Acquisition and the issuance of the Exchange Shares in the Equity Financing and the filing of this proxy statement with the SEC.
Accounting Treatment of the Acquisition
The Acquisition is expected to be accounted for as a business acquisition, with Avalon identified as the accounting acquirer. Avalon is considered the accounting acquirer since immediately following the closing: (i) Avalon stockholders will own a majority of the voting rights of the post-Acquisition company; (ii) Avalon will have designate a majority (eight of nine) of the initial members of the board of directors of the post-Acquisition company; (iii) Avalon’s senior management will hold the majority of the key positions in senior management of the post-Acquisition company; and (iv) Avalon will continue to maintain its corporate headquarters in Freehold, New Jersey, United States. SenlangBio will continue to maintain operations in the Shijiazhuang High-tech Development Zone, Hebei Province, China.
The acquisition consideration is 81,000,000 shares of Avalon Common Stock. The purchase price will be allocated to the acquired assets and assumed liabilities based on their fair values at the closing date, and any excess is initially allocated to identifiable intangible assets mainly consisting of cell and gene engineering technologies with the ability to generate innovative and transformative cellular immunotherapies for solid and hematologic cancers, which will be amortized over 10 years. The initial allocation is subject to change upon the final valuation which is to be done at the time of closing. Such change could have a material impact on Avalon’s financial statements.
Material U.S. Federal Income Tax Consequences of the Acquisition
Neither Avalon nor its stockholders are expected to recognize federal income tax or gain as a result of the Acquisition.
Ownership Following the Acquisition and the Equity Financing
It is anticipated that, upon the consummation of the Acquisition, the ownership of Avalon will be as follows:
| ● | Current Avalon stockholders will own 51.0% of the total voting shares outstanding; and |
| ● | Current Sen Lang Shareholders will own 49.0% of the total voting shares outstanding. |
The numbers of shares and percentage interests set forth above do not take into account (i) shares of Avalon Common Stock issuable upon the exchange of shares of SenlangBio purchased by the Investor in the Equity Financing, pursuant to the Exchange Agreement, (ii) shares of Avalon Common Stock issuable upon the exercise of outstanding options and warrants and (iii) potential future issuances of Avalon securities.
In addition, under the Exchange Agreement, the Investor shall have the right, exercisable following the six month anniversary of the closing of the Acquisition and until the five year anniversary of the closing of the Acquisition, to elect to exchange, from time to time, all or part of its equity ownership of SenlangBio for Exchange Shares of Avalon at an effective exchange price of $1.21 per share of Avalon Common Stock. Following the completion of the financing and assuming the full funding by the investor in the financing, the aggregate number of shares of Avalon Common Stock that would be issuable under the Exchange Agreement (assuming the exchange of all shares) would be approximately 25,885,000 (using the conversion rate of US dollars to RMB of 6.3856 as of June 11, 2021). The resultant equity ownership of Avalon would be as follows:
| ● | Current Avalon stockholders will own 44.1% of the total voting shares outstanding; |
| ● | Current Sen Lang Shareholders will own 42.4% of the total voting shares outstanding and |
| ● | The Investor will own 13.5% of the total voting shares outstanding. |
13
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Certain statements in this proxy statement may constitute “forward-looking statements” for purposes of the federal securities laws. Our forward-looking statements include, but are not limited to, statements regarding Avalon, Avalon’s management team’s, SenlangBio and SenlangBio’s management team’s expectations, hopes, beliefs, intentions or strategies regarding the future. In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intends,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “should,” “will,” “would” and similar expressions may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking. Actual results may differ materially from Avalon’s and SenlangBio’s current expectations depending upon a number of factors. These factors include, among others:
| ● | the inherent uncertainty associated with financial projections, restructuring in connection with, and successful consummation of, the Acquisition; |
| ● | subsequent integration of Avalon’s and SenlangBio’s businesses and the ability to recognize the anticipated synergies and benefits of the Acquisition; |
| ● | the inability to complete the Acquisition due to the failure to satisfy conditions to the closing in the Purchase Agreement and/or the failure the complete the Equity Financing; |
| ● | the post-Acquisition company’s financial and business performance following the Acquisition, including plans to develop and commercialize additional products; |
| ● | changes in SenlangBio’s and Avalon’s strategy, future operations, financial position, estimated revenues and losses, projected costs, prospects and plans; |
| ● | developments and projections relating to the post-Acquisition company’s competitors or industry; |
| ● | the impact of health epidemics, including the COVID-19 pandemic, on the business of Avalon and SenlangBio; |
| ● | the ability to protect and maintain the post-Acquisition company’s intellectual property protection and not infringe on the rights of others; |
| ● | developments and projections relating to the post-Acquisition company’s competitors or industry; |
| ● | future regulatory, judicial and legislative changes in Avalon’s or SenlangBio’s industry; |
| ● | access to available financing (including the Equity Financing in connection with the Acquisition) on a timely basis and on reasonable terms; |
| ● | the receipt of required regulatory approvals for the Acquisition; |
| ● | the diversion of management time on Acquisition-related issues; |
| ● | the inability to maintain the listing of Avalon Common Stock on Nasdaq following the Acquisition; |
| ● | the outcome of any legal proceedings that may be instituted against Avalon following announcement of the proposed Acquisition and transactions contemplated thereby; and |
| ● | other risks and uncertainties described in this proxy statement and in the Annual Report, including those in the sections entitled “Risk Factors.” |
These forward-looking statements are based on information available as of the date of this proxy statement, and current expectations, forecasts and assumptions, and involve a number of judgments, risks and uncertainties. Accordingly, forward-looking statements should not be relied upon as representing our views as of any subsequent date, and we do not undertake any obligation to update forward-looking statements to reflect events or circumstances after the date they were made, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws. Except as expressly required by law, including the securities laws of the United States, Avalon and SenlangBio disclaim any intent or obligation to update or revise these forward-looking statements.
14
THE ANNUAL MEETING OF AVALON STOCKHOLDERS
Avalon is furnishing this proxy statement to you as part of the solicitation of proxies by its board of directors for use at the annual meeting, and at any adjournment or postponement thereof. This proxy statement is first being furnished to Avalon’s stockholders on or about [ ], 2021. This proxy statement provides you with information you need to know to be able to vote or instruct your vote to be cast at the annual meeting of stockholders.
Date, Time and Place of the Annual Meeting
Due to the public health impact of the coronavirus outbreak (COVID-19) and to support the health and well-being of Avalon’s stockholders, the Avalon annual meeting will be held in a virtual meeting format only. The annual meeting of stockholders of Avalon will be held at [ ] [a/p].m. Eastern time, on [ ], 2021, at [LINK], or such other date, time and place to which such meeting may be adjourned or postponed, for the purpose of considering and voting upon the proposals.
On the day of the Avalon annual meeting, if you have properly registered, you may enter the annual meeting by logging in using the event password you received via email in your registration confirmation at [OTHER LINK]. You will not be able to attend the Avalon annual meeting in-person.
At the Avalon annual meeting of stockholders, Avalon will ask the Avalon stockholders to vote in favor of the following proposals:
| ● | Proposal 1—to elect the nine director nominees named in the proxy statement to hold office until the next annual meeting of stockholders and until their successors are duly elected and qualified (the “Director Election Proposal”); |
| ● | Proposal 2—to ratify the appointment of Marcum LLP as the Company’s independent auditors for the fiscal year ending December 31, 2021 (the “Auditor Proposal”); and |
| ● | Proposal 3—to approve, pursuant to the rules of the Nasdaq Stock Market, the issuance of (i) the Acquisition Shares pursuant to the terms of the Purchase Agreement and (ii) the Exchange Shares (the “Nasdaq Proposal”); |
| ● | Proposal 4—to adjourn the annual meeting, if necessary, to another time or place to solicit additional proxies if there are not sufficient votes in favor of Proposal 1 (the “Adjournment Proposal”). |
Recommendation of the Avalon Board of Directors
Avalon’s board of directors believes that each of the proposals to be presented at the annual meeting of stockholders is in the best interests of Avalon and its stockholders and unanimously recommends that its stockholders vote “FOR” each of the Avalon Proposals as further described below.
When you consider the recommendation of Avalon’s board of directors, you should keep in mind that certain of Avalon’s board of directors and officers have interests in the Acquisition that are different from, or in addition to, your interests as a stockholder. These interests include, among other things:
| ● | as of June 30, 2021, Avalon’s directors and executive officers beneficially owned approximately 66.3% of the shares of Avalon Common Stock (calculated in accordance with SEC rules that define beneficial ownership); |
| ● | all of the current executive officers of Avalon will continue in their current positions after the Acquisition, and all of the directors except for Meng Li will continue on the Avalon board after the Acquisition. In addition, On April 10, 2020, in the ordinary course of business, SenlangBio entered into a scientific research project cooperation agreement with Beijing Lu Daopei Hospital Co., Ltd., under which Beijing Lu Daopei Hospital Co., Ltd. conducts scientific research for the interest of SenlangBio on the cytoplasmic CD79a antibody gated multicolor flow cytometry monitoring CD19-CAR-T bridging allogeneic transplantation for the treatment of refractory and relapsed acute B lymphocytic leukemia. SenlangBio provides research funds in the amount of RMB 2 million to Beijing Lu Daopei Hospital Co., Ltd. Beijing Lu Daopei Hospital Co., Ltd. is a wholly-owned subsidiary of an entity whose chairman is Wenzhao Lu, the Chairman and largest shareholder of Avalon; and |
| ● | the continued indemnification of current directors and officers of Avalon and the continuation of directors’ and officers’ liability insurance after the Acquisition. |
15
You will be entitled to vote or direct votes to be cast at the annual meeting of stockholders if you owned shares of Avalon Common Stock at the close of business on [ ], 2021, which is the record date for the annual meeting of stockholders. Each share of Avalon Common Stock is entitled to one vote per share. If your shares are held in “street name” or are in a margin or similar account, you should contact your broker, bank or other nominee to ensure that votes related to the shares you beneficially own are properly counted. On the record date, there were [ ] shares of Avalon Common Stock issued and outstanding.
As of June 30, 2021, Avalon’s directors and executive officers beneficially owned 66.3% of the shares of Avalon Common Stock (calculated in accordance with SEC rules that define beneficial ownership) and owned 54,145,161 shares, 63.6% of the issued and outstanding Avalon Common Stock on such date. Although under no contractual or other obligation to do so, all of such directors and executive officers are currently expected to vote in favor of all of the Avalon Proposals, including the Nasdaq Proposal.
Each share of Avalon Common Stock that you own in your name entitles you to one vote on each of the proposals for the annual meeting of stockholders. Your one or more proxy cards show the number of shares of Avalon Common Stock that you own.
If you are a holder of record, there are different ways to vote your shares at the annual meeting of stockholders:
| ● | You can vote by completing, signing and returning the enclosed proxy card in the postage-paid envelope provided. If you hold your shares in “street name” through a bank, broker or other nominee, you will need to follow the instructions provided to you by your bank, broker or other nominee to ensure that your shares are represented and voted at the applicable annual meeting(s). If you vote by proxy card, your “proxy,” whose name is listed on the proxy card, will vote your shares as you instruct on the proxy card. If you sign and return the proxy card but do not give instructions on how to vote your shares, your shares of Avalon Common Stock will be voted as recommended by Avalon’s board of directors. With respect to proposals for the annual meeting of stockholders, that means: “FOR” each of the Avalon Proposals. |
| ● | You can vote via the Internet by following the instructions on the voting instruction form or proxy card in your proxy materials. |
| ● | You can vote via telephone by following the instructions on the voting instruction form or proxy card in your proxy materials. | |
| ● | You can attend the annual meeting and vote via live website. If you wish to vote your shares electronically at the annual meeting, there will be a live link provided during the annual meeting. You will need the virtual control number assigned to you in order to vote. |
Who Can Answer Your Questions About Voting Your Shares
If you have any questions about how to vote or direct a vote in respect of your shares of Avalon Common Stock, you may contact Avalon at 4400 Route 9 South, Suite 3100, Freehold, New Jersey 07728, telephone number (732) 780-4400.
Quorum and Vote Required for the Avalon Proposals
A quorum of Avalon’s stockholders is necessary to hold a valid meeting. The holders of at least the majority of the outstanding shares of Avalon Common Stock as of the record date, represented in person or by proxy, will constitute a quorum for the transaction of business at the Avalon annual meeting. Avalon will include proxies marked as abstentions and broker non-votes to determine the number of shares present at the Avalon annual meeting.
The approval of the Auditor Proposal, the Nasdaq Proposal and the Adjournment Proposal requires the affirmative vote of the holders of a majority of votes cast by the stockholders present in person or represented by proxy and entitled to vote thereon at the annual meeting, and the approval of the Director Election Proposal requires the affirmative vote of a plurality of the votes properly cast on the election of directors. Accordingly, abstentions and broker non-votes, if any, will have no effect on the outcome of the Director Election Proposal, the Auditor Proposal, the Nasdaq Proposal and the Adjournment Proposal.
16
Abstentions and Broker Non-Votes
If your shares of Avalon Common Stock are held by your broker as your nominee, that is, in “street name,” the enclosed voting instruction card is sent by the institution that holds your shares. Please follow the instructions included on that proxy card regarding how to instruct your broker to vote your shares.
If you do not give instructions to your broker, the question of whether your broker or nominee will still be able to vote your shares depends on whether the NYSE deems the particular proposal to be a “routine” matter and how your broker or nominee exercises any discretion they may have in the voting of the shares that you beneficially own. Brokers and nominees can use their discretion to vote “uninstructed” shares with respect to matters that are considered to be “routine,” but not with respect to “non-routine” matters. Under the rules and interpretations of the NYSE, “non-routine” matters are matters that may substantially affect the rights or privileges of stockholder, such as mergers, stockholder proposals, elections of directors (even if not contested), executive compensation (including any advisory stockholder votes on executive compensation and on the frequency of stockholder votes on executive compensation), and certain corporate governance proposals, even if management-supported.
For any Avalon Proposal that is considered a “routine” matter, your broker or nominee may vote your shares in its discretion either for or against the proposal even in the absence of your instruction. For any Avalon Proposal that is considered a “non-routine” matter for which you do not give your broker instructions, the shares will be treated as broker non-votes. “Broker non-votes” occur when a beneficial owner of shares held in street name does not give instructions to the broker or nominee holding the shares as to how to vote on matters deemed “non-routine.” Broker non-votes will not be considered to be shares “entitled to vote” at the meeting and will not be counted as having been voted on the applicable proposal. Therefore, if you are a beneficial owner and want to ensure that shares you beneficially own are voted in favor or against any or all of the Avalon Proposals, the only way you can do so is to give your broker or nominee specific instructions as to how the shares are to be voted. Avalon currently anticipates that only the Auditor Proposal is likely to be deemed routine by the NYSE.
If you have submitted a proxy to vote your shares and wish to change your vote, you may do so by:
| ● | filing with Avalon’s Secretary, a letter revoking the proxy; |
| ● | submitting another signed proxy with a later date; or |
| ● | attending the Avalon annual meeting and voting online, provided you file a written revocation with the Secretary of the annual meeting prior to the voting of such proxy. |
Appraisal or Dissenters’ Rights
No appraisal or dissenters’ rights are available to holders of shares of Avalon Common Stock in connection with the Acquisition.
Avalon will pay the cost of soliciting proxies for the annual meeting, including the costs of preparing, printing and mailing this proxy statement and the Annual Report for the Avalon annual meeting. Avalon also will reimburse banks, brokers and other custodians, nominees and fiduciaries representing beneficial owners of shares of Avalon Common Stock for their expenses in forwarding soliciting materials to beneficial owners of Avalon Common Stock and in obtaining voting instructions from those owners. Avalon’s directors, officers and employees may also solicit proxies by telephone, by facsimile, by mail, on the Internet or in person. They will not be paid any additional amounts for soliciting proxies.
17
The post-Acquisition company will face a market environment that cannot be predicted and that involves significant risks, many of which will be beyond its control. In addition to the other information contained in this proxy statement, you should carefully consider the material risks described below before deciding how to vote your shares of stock. In addition, you should consider the risks associated with the business of Avalon and SenlangBio because these risks may also affect the post-Acquisition company. You should also read and consider the other information in this proxy statement. Please see the section entitled “Where You Can Find More Information.”
Preliminary Note: references in this sub-section titled “Risks Related to SenlangBio” to “we,” “our,” “us” and similar terms refer to SenlangBio and its subsidiaries.
Risks Related to SenlangBio’s Financial Position and Capital Requirements
SenlangBio is a biopharmaceutical company that will face many risks frequently encountered by clinical-stage businesses.
Marketing approval of SenlangBio’s therapeutic product candidates requires extensive clinical testing data to support the safety and efficacy requirements needed for regulatory approval. The likelihood of success of SenlangBio’s business plan must be considered in light of the problems, substantial expenses, difficulties, complications and delays frequently encountered in connection with developing and expanding clinical-stage businesses and the regulatory and competitive environment in which SenlangBio operates. Biopharmaceutical product development is a highly speculative undertaking, involves a substantial degree of risk and is a capital-intensive business.
Accordingly, investors should consider SenlangBio’s prospects in light of the costs, uncertainties, delays and difficulties frequently encountered by companies in the clinical stages of development. Potential investors should carefully consider the risks and uncertainties that a company with a limited operating history will face. In particular, potential investors should consider that SenlangBio cannot assure you that SenlangBio will be able to:
| ● | successfully implement or execute SenlangBio’s current business plan, or that SenlangBio’s business plan is sound; | |
| ● | receive approval by regulatory agencies, including the NMPA in the PRC, of clinical trial protocols so that anticipated clinical trials commence; |
| ● | successfully complete clinical trials and obtain regulatory approval for the marketing of its product candidates; |
| ● | manufacture clinical drug product and subsequently establish a commercial drug supply; |
| ● | secure market exclusivity and/or adequate intellectual property protection for its product candidates; |
| ● | achieve broad market acceptance of its product candidates in the medical community and with third party payors and consumers; |
| ● | attract and retain an experienced management and advisory team; and |
| ● | raise sufficient funds in the capital markets to effectuate its business plan including clinical development, product development/manufacture regulatory approval and commercialization for its product candidates. |
If SenlangBio cannot successfully execute any one of the foregoing, SenlangBio’s business may not succeed and your investment will be adversely affected.
18
SenlangBio has incurred operating losses in each year since its inception and expects to continue to incur substantial losses for the foreseeable future. SenlangBio may never become profitable or, if achieved, be able to sustain profitability.
SenlangBio expects to continue to incur substantial expenses and losses without sufficient revenues unless and until SenlangBio is able to obtain regulatory approval and successfully commercialize one or more of its product candidates. To date, SenlangBio has only generated limited revenue and it expects to incur significant expense to complete its clinical programs for its product candidates in the PRC and elsewhere. SenlangBio may never be able to obtain regulatory approval for the marketing of its product candidates in any indication in the PRC or elsewhere. Even if it is able to commercialize any product candidate, there can be no assurance that it will generate significant enough revenues to ever achieve profitability. SenlangBio’s net loss for the year ended December 31, 2020 and for the three months ended March 31, 2021 was $2,442,363 and $135,280, respectively. At March 31, 2021, SenlangBio’s accumulated deficit since inception was $8,515,294.
Until SenlangBio obtains FDA approval for any of its product candidates, which is not expected until after completion of several phases of clinical trials and submission and successful review of a new drug application and associated pre-marketing approval by regulatory agencies, it expects that its research and development expenses will continue to increase as it advances its clinical trials for its product candidates. As a result, SenlangBio expects to continue to incur substantial losses for the foreseeable future, and these losses will be increasing. SenlangBio is uncertain when or if it will be able to achieve or sustain profitability. If SenlangBio achieves profitability in the future, it may not be able to sustain profitability in subsequent periods. Failure to become and remain profitable would impair SenlangBio’s ability to sustain operations and adversely affect its ability to raise capital.
SenlangBio will require additional capital to fund its operations, and if SenlangBio fails to obtain necessary financing, it may not be able to complete the development and commercialization of its product candidates. SenlangBio’s cash or cash equivalent will only fund its operations for a limited time and it will need to raise additional capital to support its development and commercialization efforts.
SenlangBio expects to spend substantial amounts to complete the development of, seek regulatory approvals for and commercialize its product candidates. Even with the expected cash proceeds of approximately $30 million resulting from the Equity Financing and the revenues generated from the SenlangBio Clinical Laboratory business, SenlangBio will require substantial additional capital to complete the development and potential commercialization of its product candidates. Avalon and SenlangBio currently expect that SenlangBio’s cash reserves, based on cash balances at March 31, 2021, together with the proceeds from the Equity Financing, will fund SenlangBio’s operations for 18 months following the closing of the Acquisition, based on current plans and assumptions.
SenlangBio is currently operating at a loss and expects its operating costs will increase significantly as it incurs costs related to the clinical trials for its product candidates. At March 31, 2021, SenlangBio had a cash and cash equivalents balance of $188,887. Currently, SenlangBio’s operations are focused on utilizing cell and gene engineering technologies to generate innovative and transformative cellular immunotherapies for solid and hematologic cancers. SenlangBio provides general laboratory testing and immunology and hematology testing services for patients and other customers in China for fees.
SenlangBio had a working capital deficit of $1,774,003 at March 31, 2021, and incurred negative cash flow from operating activities of $233,267 for the three months ended March 31, 2021. SenlangBio has a limited operating history and its continued growth is dependent upon the continuation of providing general laboratory testing and immunology and hematology testing services, and obtaining additional financing to fund future obligations and pay liabilities arising from normal business operations. In addition, the current cash balance cannot be projected to cover the operating expenses for the next twelve months from March 31, 2021. These matters raise substantial doubt about SenlangBio’s ability to continue as a going concern. The ability of SenlangBio to continue as a going concern is dependent on SenlangBio’s ability to raise additional capital, implement its business plan, and generate significant revenues. There are no assurances that SenlangBio will be successful in its efforts to generate significant revenues, maintain sufficient cash balance or report profitable operations or to continue as a going concern. If the Acquisition and Equity Financing do not close, SenlangBio plans on raising capital through bank and other borrowings to implement its business plan. However, there is no assurance these plans will be realized and that any additional financings will be available to SenlangBio on satisfactory terms and conditions, if any.
19
SenlangBio does not currently have any arrangements or credit facilities in place as a source of funds, and there can be no assurance that it will be able to raise sufficient additional capital on acceptable terms, or at all. SenlangBio or the post-Acquisition company may seek, in addition to the Equity Financing, additional capital through a combination of private and public equity offerings, debt financings and strategic collaborations. Debt financing, if obtained, may involve agreements that include covenants limiting or restricting SenlangBio’s ability to take specific actions, such as incurring additional debt, and could increase its expenses and require that its assets secure such debt.
Equity financing, if obtained, could result in dilution to existing stockholders and/or require such stockholders to waive certain rights and preferences. If such financing is not available on satisfactory terms, or is not available at all, SenlangBio may be required to delay, scale back or eliminate the development of business opportunities and its operations and financial condition may be materially adversely affected. SenlangBio can provide no assurances that any additional sources of financing will be available to it on favorable terms, if at all. In addition, if SenlangBio is unable to secure sufficient capital to fund its operations, it might have to enter into strategic collaborations that could require it to share commercial rights to its product candidates with third parties in ways that it currently does not intend or on terms that may not be favorable to SenlangBio.
Risks Related to SenlangBio’s Business
The NMPA may refuse to consider the data from the investigator-initiated clinical trials of SenlangBio due to concerns that (1) this does not follow the mainstream regulatory pathway of relying on registered clinical trials, or that (2) the non-registered clinical trials of the product may not otherwise fully comply with the same requirements applicable to registered clinical trials, as further explained below.
While investigator-initiated trials may provide us with clinical data that can inform our future development strategy, we do not have full control over the protocols, administration, or conduct of the trials. As a result, we are subject to risks associated with the way investigator-initiated trials are conducted, and there is no assurance the clinical data from any of our investigator-initiated clinical trials in China will be accepted by the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), Japanese Pharmaceuticals and Medical Devices Agency (PMDA) or other comparable regulatory authorities outside of China, for any of our product candidates. Third parties in such investigator-initiated clinical trials may not perform their responsibilities for our clinical trials on our anticipated schedule or consistent with clinical trial protocols or applicable regulations. Further, any data integrity issues or patient safety issues arising out of any of these trials would be beyond our control, yet could adversely affect our reputation and damage the clinical and commercial prospects for our product candidates. Additional risks include difficulties or delays in communicating with investigators or administrators, procedural delays and other timing issues, and difficulties or differences in interpreting data. Third-party investigators may design clinical trials with clinical endpoints that are more difficult to achieve, or in other ways that increase the risk of negative clinical trial results compared to clinical trials that we may design on our own. As a result, our lack of control over the design, conduct and timing of, and communications with the FDA, NMPA, EMA and PMDA regarding investigator-initiated trials expose us to additional risks and uncertainties, many of which are outside our control, and the occurrence of which could adversely affect the prospects for our product candidates.
Furthermore, there is no assurance the clinical data from any of our investigator-initiated clinical trials in China, where the patients are predominately of Chinese descent, will produce similar results in patients of different races, ethnicities or those of non-Chinese descent.
All material aspects of the research, development, manufacturing and commercialization of biopharmaceutical products in China are heavily regulated. Any failure to comply with existing regulations and industry standards, or any adverse actions by the NMPA or other comparable regulatory authorities against us, could negatively impact our reputation and our business, financial condition, results of operations and prospects.
The process of obtaining regulatory approvals and compliance with appropriate laws and regulations requires the expenditure of substantial time and financial resources. Failure to comply with the applicable requirements at any time during the product development or approval process, or after approval, may subject an applicant to administrative or judicial sanctions. These sanctions could include the regulator’s refusal to approve pending applications, withdrawal of an approval, license revocation, a clinical hold, or total or partial suspension of production or distribution. Failure to comply with these regulations could have a material adverse effect on our business.
20
Our preclinical programs may experience delays or may never advance to clinical trials, which would adversely affect our ability to obtain regulatory approvals or commercialize these product candidates on a timely basis or at all, which would have an adverse effect on our business.
Our product candidates are still in the preclinical development stage, and the risk of failure of preclinical programs is high. Before we can commence clinical trials for a product candidate, we must complete extensive preclinical testing and studies to obtain regulatory clearance to initiate human clinical trials. We cannot be certain of the timely completion or outcome of our preclinical testing and studies and cannot predict if the NMPA will accept our proposed clinical programs or if the outcome of our preclinical testing and studies will ultimately support the further development of our programs. As a result, we cannot be sure that we will be able to submit IND applications for all of our preclinical programs on the timelines we expect, and we cannot be sure that submission of IND applications will result in the NMPA allowing clinical trials to begin.
Clinical trials are difficult to design and implement, involve uncertain outcomes and may not be successful.
Human clinical trials are difficult to design and implement, in part because they are subject to rigorous regulatory requirements. The design of a clinical trial can determine whether its results will support approval of a product candidate and flaws in the design of a clinical trial may not become apparent until the clinical trial is well advanced. As an organization, we have limited experience designing clinical trials and may be unable to design and execute clinical trials to support regulatory approval. There is a high failure rate for biologic products proceeding through clinical trials, which may be higher for our product candidates because they are based on new technology and engineered on a patient-by-patient basis. Many companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in late-stage clinical trials even after achieving promising results in preclinical testing and earlier-stage clinical trials. Data obtained from preclinical and clinical activities are subject to varying interpretations, which may delay, limit or prevent regulatory approval. In addition, we may experience regulatory delays or rejections as a result of many factors, including changes in regulatory policy during the period of our product candidate development. Any such delays could negatively impact our business, financial condition, results of operations and prospects.
Success in preclinical studies or clinical trials may not be indicative of results in future clinical trials.
Results from preclinical studies are not necessarily predictive of future clinical trial results, and interim results of a clinical trial are not necessarily indicative of final results. While we have received some positive data in previous preclinical and IIT trials, we are still in the process of producing and gathering more data and are still conducting more additional preclinical and IIT trials in order to seek regulatory approvals. For that reason, we do not know whether these candidates will be effective and safe for the intended indications in humans. Our product candidates may fail to show the desired safety and efficacy in further, registered clinical development despite positive results in preclinical and IIT studies. This failure to establish sufficient efficacy and safety could cause us to abandon clinical development of our product candidates.
We depend on enrollment of patients in our clinical trials for our product candidates. If we encounter difficulties enrolling patients in our clinical trials, our clinical development activities could be delayed or otherwise adversely affected.
Identifying and qualifying patients to participate in clinical trials of our product candidates is critical to our success. We may experience difficulties in patient enrollment in our clinical trials for a variety of reasons. The timely completion of clinical trials in accordance with the protocols depends, among other things, on our ability to enroll a sufficient number of patients who remain in the study until its conclusion. The enrollment of patients depends on many factors, including:
| ● | the patient eligibility criteria defined in the protocol; | |
| ● | the number of patients with the disease or condition being studied; | |
| ● | the understanding of risks and benefits of the product candidate in the trial; | |
| ● | clinicians’ and patients’ perceptions as to the potential advantages of the product candidate being studied in relation to other available therapies, including any new drugs that may be approved for the indications we are investigating or drugs that may be used off-label for these indications; |
21
| ● | the size and nature of the patient population who meet inclusion criteria; | |
| ● | the proximity of patients to study sites; | |
| ● | the design of the clinical trial; | |
| ● | clinical trial investigators’ ability to recruit clinical trial investigators with the appropriate competencies and experience; | |
| ● | competing clinical trials for similar therapies or other new therapeutics not involving T cell-based immunotherapy; | |
| ● | our ability to obtain and maintain patient consents; and | |
| ● | the risk that patients enrolled in clinical trials will drop out of the clinical trials before completion of their treatment. |
Delays in patient enrollment may result in increased costs or may affect the timing or outcome of the planned clinical trials, which could prevent completion of these clinical trials and adversely affect our ability to advance the development of our product candidates. In addition, many of the factors that may lead to a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
If the clinical trials of any of our product candidates fail to demonstrate safety and efficacy to the satisfaction of the NMPA, or do not otherwise produce favorable results, we may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of our product candidates.
We may not commercialize, market, promote or sell any product candidate without obtaining marketing approval from the NMPA, and we may never receive such approvals. It is impossible to predict accurately when or if any of our product candidates will prove effective or safe in humans and will receive regulatory approval. Before obtaining marketing approval from regulatory authorities for the commercial sale of any of our product candidates, we must demonstrate through lengthy, complex and expensive preclinical studies and clinical trials that our product candidates are both safe and effective for use in each proposed indication. Clinical trials are expensive, difficult to design and implement, can take many years to complete and are uncertain as to outcome. A failure of one or more clinical trials can occur at any stage of clinical development.
We may experience numerous unforeseen events prior to, during or as a result of clinical trials that could delay or prevent our ability to receive marketing approval or commercialize any of our product candidates, including:
| ● | the NMPA may disagree as to the number, design or implementation of our clinical trials, or may not interpret the results from clinical trials as we do; | |
| ● | regulators may not authorize us or our investigators to commence a clinical trial or conduct a clinical trial at a prospective trial site; | |
| ● | we may not reach agreement on acceptable terms with prospective clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different clinical trial sites; | |
| ● | clinical trials of our product candidates may produce negative or inconclusive results; | |
| ● | we may decide, or regulators may require us, to conduct additional clinical trials or abandon product development programs; | |
| ● | the number of patients required for clinical trials of our product candidates may be larger than we anticipate, enrollment in these clinical trials may be slower than we anticipate, participants may drop out of these clinical trials at a higher rate than we anticipate or we may fail to recruit eligible patients to participate in a trial; | |
| ● | our third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all; |
22
| ● | regulators may issue a clinical hold, or regulators may require that we or our investigators suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements or a finding that the participants are being exposed to unacceptable health risks; | |
| ● | the cost of clinical trials of our product candidates may be greater than we anticipate; | |
| ● | the NMPA may fail to approve our manufacturing processes or facilities; |
| ● | the supply or quality of our product candidates or other materials necessary to conduct clinical trials of our product candidates may be insufficient or inadequate; | |
| ● | our product candidates may have undesirable side effects or other unexpected characteristics, particularly given their novel, first-in-human application, causing us or our investigators, or regulators to suspend or terminate the clinical trials; and | |
| ● | the approval policies or regulations of the NMPA may significantly change in a manner rendering our clinical data insufficient for approval. |
To the extent that the results of the trials are not satisfactory for the NMPA to approve our new drug application, the commercialization of our product candidates may be significantly delayed, or we may be required to expend significant additional resources, which may not be available to us, to conduct additional trials in support of potential approval of our product candidates.
Coronavirus could adversely impact SenlangBio’s business, including SenlangBio’s clinical trials.
In December 2019, a novel strain of coronavirus, COVID-19, was reported to have surfaced in Wuhan, China. Since then, the COVID-19 coronavirus has spread globally. The outbreak and government measures taken in response have also had a significant impact, both direct and indirect, on businesses, as worker shortages have occurred; supply chains have been disrupted; facilities and production have been suspended; and demand for certain goods and services, such as medical services and supplies, has spiked. As a result, we may experience disruptions that could severely impact our business and clinical trials, including:
| ● | delays or difficulties in enrolling patients in planned clinical trials; |
| ● | delays or difficulties in clinical site initiation, including difficulties in recruiting clinical site investigators and clinical site staff; |
| ● | diversion of healthcare resources away from the conduct of clinical trials, including the diversion of hospitals serving as clinical trial sites and hospital staff supporting the conduct of SenlangBio’s clinical trials; |
| ● | interruption of key clinical trial activities, such as clinical trial site monitoring, due to limitations on travel imposed or recommended by federal or state governments, employers and others; |
| ● | limitations in employee resources that would otherwise be focused on the conduct of clinical trials, including because of sickness of employees or their families or the desire of employees to avoid contact with large groups of people; |
| ● | delays in receiving approval from local regulatory authorities to initiate planned clinical trials; |
| ● | delays in clinical sites receiving the supplies and materials needed to conduct clinical trials; |
| ● | interruption in global shipping that may affect the transport of clinical trial materials, such as investigational drug product used in clinical trials; |
| ● | changes in local regulations as part of a response to the COVID-19 coronavirus outbreak which may require changes in the ways in which clinical trials are conducted, which may result in unexpected costs, or to discontinue the clinical trials altogether; and |
| ● | delays in necessary interactions with local regulators, ethics committees and other important agencies and contractors due to limitations in employee resources or forced furlough of government employees. |
23
The global outbreak of the COVID-19 coronavirus continues to rapidly evolve. The extent to which the COVID-19 coronavirus may impact SenlangBio’s business and clinical trials will depend on future developments, which are highly uncertain and cannot be predicted with confidence, such as the ultimate geographic spread of the disease, the duration of the outbreak, travel restrictions and social distancing in the PRC and other countries, business closures or business disruptions and the effectiveness of actions taken in the PRC and other countries to contain and treat the disease. The COVID-19 pandemic could negatively impact SenlangBio’s operations even though the pandemic did not significantly impact its operation in the past. However, given the dynamic nature of these circumstances, the uncertainty around the potential resurgence of the COVID-19 cases in China, and the instability of local policies and restrictions, the COVID-19 impact over SenlangBio’s business in the rest of year 2021 cannot be reasonably estimated at this time. If COVID-19 cases resurged in the area SenlangBio conducted its business and local government implemented new restrictions in the effort to contain the spread, it is expected SenlangBio’s business will be negatively impacted.
SenlangBio faces competition from other biotechnology and pharmaceutical companies and its operating results will suffer if SenlangBio fails to compete effectively.
The biotechnology and pharmaceutical industries are intensely competitive and subject to rapidly evolving technology and intense research and development efforts. SenlangBio has competitors in a number of jurisdictions that have substantially greater name recognition, commercial infrastructures and financial, technical and personnel resources than it has. Established competitors may invest heavily to quickly discover and develop novel compounds that could make SenlangBio’s product candidates obsolete or uneconomical. Any new product that competes with an approved product may need to demonstrate compelling advantages in efficacy, cost, convenience, tolerability and safety to be commercially successful. Other competitive factors, including generic competition, could force SenlangBio to lower prices or could result in reduced sales. In addition, new products developed by others could emerge as competitors to SenlangBio’s product candidates. If SenlangBio is not able to compete effectively against its current and future competitors, its business will not grow and its financial condition and operations will suffer.
The competitive landscape includes companies that are engaging in cell and gene therapies. Considering the CAR-T field, some notable competitors (in similar size and scale of business) include (but not limited to) Gracell Biotechnologies (NASDAQ: GRCL), Legend Biotech Corporation (NASDAQ: LEGN), and Poseida Therapeutics, Inc. (NASDAQ: PSTX). For CAR-GDT cell therapy field, some notable companies include (but not limited to) Adicet Bio Inc. (NASDAQ: ACET), Incysus Therapeutics, Inc and GammaDelta Therapeutics.
Risks Relating to SenlangBio’s Intellectual Property Rights
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and patent applications are due to be paid to the NIPA (National Intellectual Property Administration) in several stages over the lifetime of a patent. NIPA requires compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. Although an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which non-compliance can result in abandonment, loss of priority or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the PRC. Non-compliance events that could result in abandonment or lapse of a patent or patent application include failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. In any such event, our competitors or other third parties might be able to enter the market, which would have a material adverse effect on our competitive position, business, financial condition, result of operations and prospects.
24
Changes in patent law could extend the expected expiry date of third party patents.
In China, intellectual property laws are constantly evolving, with efforts being made to improve intellectual property protection in China. For example, an Amendment to the PRC Patent Law is implemented on June 1 2021 and proposed to introduce patent extensions to patents of new drugs that launched in the PRC. If adopted, patents owned by third parties may be extended, which may in turn affect our ability to commercialize our products without facing infringement risks. The adoption of this draft amendment may enable the patent owner to submit applications for a patent term extension. The length of any such extension is uncertain. If we are required to delay commercialization for an extended period of time, technological advances may develop and new products may be launched, which may in turn render our products non-competitive. We cannot guarantee that any other changes to PRC intellectual property laws would not have a negative impact on our intellectual property protection.
Intellectual property rights do not necessarily address all potential threats.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect our business or permit us to maintain our competitive advantage. For example:
| ● | others may be able to make products that are similar to any product candidates we may develop or utilize similar technology that are not covered by the claims of the patents that we own or license now or in the future; |
| ● | we or any of our future licensors and collaborators might not have been the first to make the inventions covered by the issued patent or pending patent application that we own or may license in the future; | |
| ● | we or any of our future licensors and collaborators might not have been the first to file patent applications covering certain of our or their inventions; | |
| ● | others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing, misappropriating or otherwise violating our intellectual property rights; | |
| ● | it is possible that our pending owned or licensed patent applications will not lead to issued patents; | |
| ● | patents that we hold rights to or that may be issued from our pending patent applications may not provide us with a competitive advantage, or may be held invalid or unenforceable, including as a result of legal challenges by our competitors or third parties; | |
| ● | our competitors or other third parties might conduct research and development activities in jurisdictions where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets; | |
| ● | we may obtain patents for certain inventions many years before we obtain marketing approval for products containing such compounds, and because patents have a limited life, which may begin to run prior to the commercial sale of the related product, the commercial value of our patents may be limited; | |
| ● | we may not develop additional proprietary technologies that are patentable; | |
| ● | the patents of others may harm our business; and | |
| ● | we may choose not to file a patent for certain trade secrets or know-how, and a third party may subsequently file a patent covering such intellectual property. |
Should any of these events occur, they could have a material adverse effect on our business, financial condition, results of operations and prospects.
25
Even if we are able to obtain patent protection for our product candidates, the life of such protection, if any, is limited, and third parties could be able to circumvent our patents by developing similar or alternative products and technologies in a non-infringing manner, or develop and commercialize products and technologies similar or identical to ours and compete directly against us after the expiration of our patent rights, if any, and our ability to successfully commercialize any product or technology would be materially adversely affected.
The life of a patent and the protection it affords is limited. For example, in China, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its filing date. Even if we successfully obtain patent protection for an approved product candidate, it may face competition from generic or biosimilar medications. Manufacturers of generic or biosimilar drugs may challenge the scope, validity or enforceability of our patents in court or before a patent office, and we may not be successful in enforcing or defending those intellectual property rights and, as a result, may not be able to develop or market the relevant product exclusively, which would materially adversely affect any potential sales of that product.
General SenlangBio-Related Risks
If SenlangBio is not successful in attracting and retaining highly qualified personnel, it may not be able to successfully implement its business strategy. In addition, the loss of the services of certain key employees, including, Jianqiang Li, Ph.D., SenlangBio’s Scientific Founder and Chief Scientific Officer and Shengmin Guo, Co-founder and Chief Executive Officer, would adversely impact SenlangBio’s business prospects.
SenlangBio’s ability to compete in the highly competitive pharmaceuticals industry depends in large part upon its ability to attract highly qualified managerial, scientific and medical personnel.
SenlangBio’s management team has expertise in many different aspects of drug development and commercialization. However, it will need to hire additional personnel as it further develops its product candidates. Competition for skilled personnel in China is intense and competition for experienced scientists may limit SenlangBio’s ability to hire and retain highly qualified personnel on acceptable terms. Despite its efforts to retain valuable employees, members of its management, scientific and medical teams may terminate their employment with SenlangBio on short notice (30 days under PRC law). SenlangBio entered into an employment agreement with Dr. Li on September 1, 2017. The loss of the services of any of SenlangBio’s executive officers or other key employees could potentially harm its business, operating results or financial condition. In particular, SenlangBio believes that the loss of the services of Dr. Li and Ms. Guo would have a material adverse effect on its business. SenlangBio’s success also depends on its ability to continue to attract, retain and motivate highly skilled junior, mid-level, and senior managers as well as junior, mid-level, and senior scientific and medical personnel.
Other biopharmaceutical companies with which SenlangBio competes for qualified personnel have greater financial and other resources, different risk profiles, and a longer history in the industry than it does. They also may provide more diverse opportunities and better chances for career advancement. Some of these characteristics may be more appealing to high-quality candidates than what SenlangBio has have to offer. If SenlangBio is unable to continue to attract and retain high-quality personnel, the rate and success at which it can develop and commercialize product candidates would be limited.
Risks Related to the VIE Structure and SenlangBio being a PRC Domestic Entity
The business of SenlangBio may fall into the prohibited foreign investment category under currently effective PRC laws.
On March 15, 2019, the NPC promulgated the Foreign Investment Law, which took effect on January 1, 2020 and replaced three existing laws regulating foreign investment in China, namely, the PRC Equity Joint Venture Law, the PRC Cooperative Joint VentureLaw and the Wholly Foreign-owned Enterprise Law, together with their implementation rules and ancillary regulations. The Foreign Investment Law grants foreign invested entities the same treatment as PRC domestic entities, except for those foreign invested entities that operate in industries deemed to be either “restricted” or “prohibited” in the “negative list” published by the State Council. Sen Lang is a BVI company and the PRC Subsidiary is currently considered to be a foreign invested entity.
26
The latest version of the “negative list,” namely, the Special Management Measures (Negative List) for the Access of Foreign Investment (2019), which became effective on July 30, 2019, provides that foreign investment is prohibited in the development and application of genetic diagnosis and treatment technology. However, the PRC laws do not clarify the meaning of “development and application of genetic diagnosis and treatment technology” and do not explain whether transactions involving a VIE Structure should be considered as “investment” in the context of the prohibition of foreign investment. SenlangBio’s main business is conducting R&D and clinical transformation of immunotherapy cell therapy, which involves modifying the patient’s T-Cells genetically. Despite the foregoing lack of clarity, the applicable rules could be interpreted in a way unfavorable to the business of SenlangBio. First, in the context of law enforcement, if the competent PRC authorities and courts interpret “development and application of genetic diagnosis and treatment technology” broadly, the modification of T-Cells genetically could be considered as falling into the prohibited foreign investment category. Second, the newly implemented foreign investment law of China includes a catch-all definition of “foreign investment” that covers any other types of investment into China besides those listed in the law, which is generally believed as a strategy to leave room for the implementation of rules to address the VIE Structure. Therefore, the VIE Structure could be considered a violation of the applicable PRC laws.
There can be no assurance that the PRC government will ultimately take a view that is not contrary to Avalon and SenlangBio’s view that the parties are complying with PRC law. If SenlangBio’s CAR-T cell therapies or other technologies that are being researched and developed are deemed by relevant PRC regulatory agencies as falling into the category of “genetic diagnosis and treatment technology,” SenlangBio would be prohibited from engaging in the research or development of such technologies. In that event, Avalon and the Sen Lang Beneficial Shareholders would have to restructure Avalon’s control over SenlangBio. SenlangBio may also have to forfeit its income derived from the research and development of such technologies. Any of these occurrences may harm Avalon’s and SenlangBio’s business, prospects, financial condition and results of operations significantly.
The filing or change of the medical institution practice license of SenlangBio Clinical Laboratory may be affected by the VIE Structure.
As SenlangBio Clinical Laboratory is a medical institution under the PRC laws, its operation is subject to the PRC regulation of foreign investment in the area of medical institution, which provides that a foreign investor can acquire 70% (to the highest extent) of the equity interests in a PRC medical institution. The relevant PRC laws also provide that the related government authority shall not approve any application of licenses/permits if the application is related to a company failing to comply with PRC foreign investment regulation.
Therefore, if the competent PRC authority responsible for the registration of the medical institution practice license of SenlangBio Clinical Laboratory adopts a broad understanding of foreign investment rules that controlling via agreements can be deemed as a way of investment, the authority may disapprove SenlangBio Clinical Laboratory’s application in relation to its medical institution practice license, including any extension of such license. In the worst case, theoretically, the competent authorities may deem the VIE Agreements unenforceable because they are in violation of the PRC laws. In that event, SenlangBio Clinical Laboratory would not be qualified to conduct any business of testing of immunology, serology and molecular genetics specialties for patients, including hematology-tumor diagnostics and testing prior to clinical trials for cell therapy, which would result in the loss of the license and thereby the loss of income to SenlangBio from this business.
Risks Related to the Acquisition
The amount of Acquisition Shares being issued to the Sen Lang Shareholders will not be adjusted in the event of any change in Avalon’s stock price.
The aggregate number of Acquisition Shares being issued to the Sen Lang Shareholders is fixed. Therefore, the total value of the Acquisition Shares will depend on the market price of the Avalon Common Stock at the closing of the Acquisition.
The market price of the Avalon Common Stock has fluctuated in the past, may fluctuate upon the announcement of the Purchase Agreement and may continue to fluctuate through the closing of the Acquisition and thereafter. The total market value of the Acquisition Shares to be issued at the closing will not be known until that time. Therefore, current and historical market prices of Avalon Common Stock may not reflect the value that the Sen Lang Shareholders will receive in the Acquisition, and the current stock price quotations for Avalon Common Stock may not provide meaningful information to Avalon stockholders. Avalon Common Stock is traded on The Nasdaq Capital Market under the symbol “AVCO.” Changes in the market price of Avalon Common Stock may result from a variety of factors that are beyond the control of Avalon, including changes in its business, operations and prospects, regulatory considerations, governmental actions, and legal proceedings and developments. You are urged to obtain up-to-date prices for Avalon Common Stock.
27
Failure to complete the Acquisition could negatively impact the stock price and the future business and financial results of Avalon.
The parties’ respective obligations to complete the Acquisition are subject to the satisfaction or waiver of a number of conditions set forth in the Purchase Agreement and described elsewhere in this proxy statement. There can be no assurance that the conditions to completion of the Acquisition will be satisfied or waived or that the Acquisition will be completed. If the Acquisition is not completed for any reason, the ongoing business of Avalon may be materially and adversely affected and, without realizing any of the benefits of having completed the Acquisition, Avalon would be subject to a number of risks, including the following:
| ● | Avalon may experience negative reactions from the financial markets, including negative impacts on the trading price of Avalon Common Stock, which could affect Avalon’s ability to secure sufficient financing in the future on attractive terms (or at all), and from its customers, vendors, regulators and employees; | |
| ● | Avalon will be required to pay its expenses incurred in connection with the Acquisition, whether or not the Acquisition is completed; | |
| ● | matters relating to the Acquisition (including integration planning) will require substantial commitments of time and resources by Avalon management and the expenditure of significant funds in the form of fees and expenses, which would otherwise have been devoted to day-to-day operations and other opportunities that may have been beneficial to Avalon otherwise. |
If any of these risks materialize, they may materially and adversely affect Avalon’s business, financial condition, financial results and stock prices.
The Acquisition is subject to a number of closing conditions and, if these conditions are not satisfied, the Purchase Agreement may be terminated in accordance with its terms and the Acquisition may not be completed. In addition, the parties have the right to terminate the Purchase Agreement under other specified circumstances, in which case the Acquisition would not be completed.
The Acquisition is subject to a number of closing conditions and, if these conditions are not satisfied or waived (to the extent permitted by law), the Acquisition will not be completed. These conditions include, among others: (i) the absence of certain legal impediments, (ii) obtaining all governmental authorizations, (iii) obtaining the Avalon stockholders’ approval, (iv) the consummation of the Equity Financing, (v) the execution of the VIE Agreements and (vi) the listing of the Acquisition Shares and the Exchange Shares on Nasdaq. In addition, each party’s obligation to complete the Acquisition is subject to the accuracy of the other parties’ representations and warranties in the Purchase Agreement (subject in most cases to “material adverse effect” qualifications), the other parties’ compliance, in all material respects, with their respective covenants and agreements in the Purchase Agreement.
The conditions to the closing may not be fulfilled and, accordingly, the Acquisition may not be completed. In addition, if the Acquisition is not completed by December 31, 2021, any party may choose not to proceed with the Acquisition. Moreover, the parties can mutually decide to terminate the Purchase Agreement at any time prior to the consummation of the Acquisition, before or after receipt of the Avalon stockholders’ approval and each party may elect to terminate the Purchase Agreement in certain other circumstances, as described in the Purchase Agreement.
28
There can be no assurance that Avalon will be able to complete the Equity Financing.
It is a condition to closing of the Acquisition that the Equity Financing shall have been consummated prior to or contemporaneously with the closing. The closing of the Equity Financing pursuant to the OpCo Capital Increase Agreement is subject to various conditions to closing, as well as the risk that the Investor does not abide by the terms of the OpCo Capital Increase Agreement. If the Equity Financing does not close for any reason, Avalon would need to waive or agree to amend the applicable condition to closing of the Acquisition. In addition, if the Equity Financing was not consummated and the Acquisition closed nevertheless, Avalon may not have sufficient capital for all planned expenses of the post-Acquisition company, and would likely need to raise additional capital. In such event, Avalon would likely seek to sell common or preferred equity or convertible debt securities, enter into a credit facility or another form of third-party funding, or seek other debt financing. The sale of equity and convertible debt securities may result in dilution to our stockholders and certain of those securities may have rights senior to those of the holders of Avalon Common Stock. If we raise additional funds through the issuance of preferred stock, convertible debt securities or other debt financing, these securities or other debt could contain covenants that would restrict its operations, fund raising capabilities or otherwise. The source, timing and availability of any future financing will depend principally upon market conditions, and, more specifically, on the progress of our clinical development programs following the Acquisition. Funding may not be available when needed, at all, or on terms acceptable to us. Lack of necessary funds may require us among other things, to delay, scale back or eliminate some or all of our planned clinical trials.
Avalon may not realize the anticipated benefits of the Acquisition.
While Avalon will continue to operate as in the past until the completion of the Acquisition, the success of the Acquisition will depend, in part, on Avalon’s ability to realize the anticipated benefits acquiring Sen Lang’s business. Avalon’s ability to realize these anticipated benefits is subject to certain risks, including, among others:
| ● | Avalon’s ability to successfully integrate the Sen Lang business; | |
| ● | the risk that Sen Lang’s business will not perform as expected; | |
| ● | the extent to which the parties will be able to realize the expected synergies, which include taking advantage of Sen Lang’s manufacturing and laboratory facilities and geographic location in Northern China; | |
| ● | the possibility that the aggregate consideration being paid for Sen Lang is greater than the value Avalon will derive from the Acquisition; | |
| ● | the reduction of cash available for operations and other uses; | |
| ● | the assumption of known and unknown liabilities of Sen Lang; and | |
| ● | the possibility of costly litigation challenging the Acquisition. |
Integrating Avalon’s and Sen Lang’s businesses may be more difficult, time-consuming or costly than expected.
Avalon and Sen Lang have operated and, until completion of the Acquisition will continue to operate, independently, and there can be no assurances that their businesses can be integrated successfully. It is possible that the integration process could result in the loss of key employees, the disruption of either company’s or both companies’ ongoing businesses or unexpected integration issues, such as higher than expected integration costs and an overall post-completion integration process that takes longer than originally anticipated. Specifically, issues that must be addressed in integrating the operations of Avalon and Sen Lang in order to realize the anticipated benefits of the Acquisition so the business performs as expected include, among others:
| ● | combining the companies’ separate operational, financial, reporting and corporate functions; |
| ● | integrating the companies’ technologies, products and services; | |
| ● | identifying and eliminating redundant and underperforming operations and assets; |
29
| ● | harmonizing the companies’ operating practices, employee development, compensation and benefit programs, internal controls and other policies, procedures and processes; | |
| ● | addressing possible differences in corporate cultures and management philosophies; | |
| ● | maintaining employee morale and retaining key management and other employees; | |
| ● | attracting and recruiting prospective employees; | |
| ● | consolidating the companies’ corporate, administrative and information technology infrastructure; | |
| ● | coordinating sales, distribution and marketing efforts; | |
| ● | managing the movement of certain businesses and positions to different locations; | |
| ● | maintaining existing agreements with customers and vendors and avoiding delays in entering into new agreements with prospective customers and vendors; | |
| ● | coordinating geographically dispersed organizations; and | |
| ● | effecting potential actions that may be required in connection with obtaining regulatory approvals. |
In addition, at times, the attention of certain members of each company’s management and each company’s resources may be focused on completion of the Acquisition and the integration of the businesses of the two companies and diverted from day-to-day business operations, which may disrupt each company’s ongoing business and, consequently, the business of the Company.
Avalon and Sen Lang will be subject to business uncertainties and contractual restrictions while the Acquisition is pending.
Uncertainty about the effect of the Acquisition on employees, vendors and customers may have an adverse effect on Avalon or Sen Lang and consequently on us after the closing of the Acquisition. These uncertainties may impair Avalon’s and Sen Lang’s ability to retain and motivate key personnel and could cause customers and others that deal with Avalon and Sen Lang, as applicable, to defer or decline entering into contracts with Avalon or Sen Lang, as applicable, or making other decisions concerning Avalon or Sen Lang, as applicable, or seek to change existing business relationships with Avalon or Sen Lang, as applicable. In addition, if key employees depart because of uncertainty about their future roles and the potential complexities of the Acquisition, Avalon’s and Sen Lang’s businesses could be harmed. Furthermore, the Purchase Agreement places certain restrictions on the operation of Avalon’s and Sen Lang’s businesses prior to the closing of the Acquisition, which may delay or prevent Avalon and Sen Lang from undertaking certain actions or business opportunities that may arise prior to the consummation of the Acquisition. Please see the section entitled “The Purchase Agreement—Covenants; Conduct of Business Pending the Acquisition” for a description of the restrictive covenants applicable to Avalon and Sen Lang.
Third parties may terminate or alter existing contracts or relationships with Avalon or Sen Lang.
Each of Avalon and Sen Lang has contracts with customers, vendors and other business partners which may require Avalon or Sen Lang, as applicable, to obtain consents from these other parties in connection with the Acquisition. If these consents cannot be obtained, the counterparties to these contracts and other third parties with which Avalon and/or Sen Lang currently have relationships may have the ability to terminate, reduce the scope of or otherwise materially adversely alter their relationships with either party in anticipation of the Acquisition, or with us following the Acquisition. The pursuit of such rights may result in Avalon and Sen Lang suffering a loss of potential future revenue, incurring liabilities in connection with a breach of such agreements or losing rights that are material to its business. Any such disruptions could limit our ability to achieve the anticipated benefits of the Acquisition. The adverse effect of such disruptions could also be exacerbated by a delay in the completion of the Acquisition or the termination of the Acquisition.
30
Avalon or Sen Lang may waive one or more of the closing conditions to the Acquisition without re-soliciting stockholder approval.
Each of Avalon and Sen Lang has the right to waive certain of the closing conditions to the Acquisition. Any such waiver may not require re-solicitation of stockholders, in which case stockholders of Avalon will not have the chance to change their votes as a result of any such waiver and Avalon will have the ability to complete the Acquisition without seeking further stockholder approval. Any determination whether to waive any condition to the Acquisition, whether stockholder approval would be re-solicited as a result of any such waiver or whether the proxy statement would be amended as a result of any waiver will be made Avalon at the time of such waiver based on the facts and circumstances as they exist at that time, and any such waiver could have an adverse effect on us.
Avalon’s stockholders will have a reduced ownership and voting interest after the Acquisition and the Equity Financing and will exercise less influence over management.
After the completion of the Acquisition and the Equity Financing, Avalon’s stockholders will own a smaller percentage of the Company than they currently own. Upon completion of the Acquisition, it is expected that Avalon stockholders will own 51.0% of the total voting shares outstanding of Avalon, and the Sen Lang Shareholders will own 49.0% of the total voting shares outstanding of Avalon, in each case immediately after consummation of the Acquisition and not giving effect to the closing of the Equity Financing. As of the result of the Equity Financing and depending on the amount of shares of OpCo that the Investor elects to exchange for shares of Avalon Common Stock pursuant to the terms of the Exchange Agreement, the percentages set forth above will be reduced proportionately by the Exchange Shares. Consequently, Avalon stockholders, as a group, will have reduced ownership and voting power in us compared to their current ownership and voting power in Avalon and, therefore, will be able to exercise less collective influence over the management and policies of Avalon than they currently exercise.
Executive officers and directors of Avalon may have interests in the Acquisition that are different from, or in addition to, the rights of Avalon stockholders.
Executive officers of Avalon negotiated the terms of the Purchase Agreement, and the Avalon board of directors approved the Purchase Agreement and the Acquisition. These executive officers and directors may have interests in the Acquisition that are different from, or in addition to, the Avalon stockholders. These interests include the continued employment of certain executive officers of Avalon following the Acquisition, the continued service of certain directors following the Acquisition and the indemnification of Avalon executive officers and directors by Avalon. Avalon stockholders should be aware of these interests when they consider their board of directors’ recommendation that stockholders vote in favor of the Avalon Proposals. For a description of the interests of Avalon’s executive officers and directors in the Acquisition, please see the section entitled “The Acquisition—Interests of Avalon’s Directors and Officers in the Acquisition.”
Any issuance of Exchange Shares could cause dilution to then existing Avalon stockholders and may depress the market price of Avalon Common Stock, and the amount of Exchange Shares issuable is impacted by the RMB to US dollar exchange rate at the time of an exchange.
Under the Exchange Agreement, the Investor shall have the right, exercisable following the six month anniversary of the closing of the Acquisition and until the five year anniversary of the closing of the Acquisition, to elect to exchange, from time to time, all or part of its equity ownership of the OpCo for Exchange Shares of Avalon at an effective exchange price of $1.21 per share of Avalon Common Stock. Following the completion of the financing and assuming the full funding by the investor in the financing, the aggregate number of shares of Avalon Common Stock that would be issuable under the Exchange Agreement (assuming the exchange of all shares) would be approximately 25,885,000 (using the conversion rate of US dollars to RMB of 6.3856 as of June 11, 2021). The issuance of Exchange Shares could result in immediate and substantial dilution to the interests of holders of Avalon Common Stock at the time of any exchanges.
In addition, the Investor is entitled to receive a number of Exchange Shares equal to the portion of the Subscription Amount (which is measured in RMB) being exchanged (i) converted into US dollars at the RMB exchange rate on the date of the exchange notice and (ii) divided by the exchange price of $1.21. Therefore, the amount of Exchange Shares issuable upon exchanges of equity of the OpCo may vary based on changes in the RMB-US dollar exchange rate during the period in which exchanges may be made under the Exchange Agreement.
31
Avalon and Sen Lang may have difficulty attracting, motivating and retaining executives and other key employees in light of the proposed Acquisition.
Our success after the Acquisition will depend in part on each of Avalon’s and Sen Lang’s ability to retain key executives and other employees. Uncertainty about the effect of the Acquisition on Avalon’s and Sen Lang’s employees may have an adverse effect on each company separately and consequently, the combined business. This uncertainty may impair our ability to attract, retain and motivate key personnel. Employee retention may be particularly challenging during the pendency of the Acquisition, as Avalon’s and Sen Lang’s employees may experience uncertainty about their future roles in the combined business.
Furthermore, if any of Avalon or Sen Lang’s key employees depart or are at risk of departing, including because of issues relating to the uncertainty and difficulty of integration, financial security or a desire not to become employees of the combined business, Avalon or Sen Lang, as applicable, may have to incur significant costs in retaining such individuals or in identifying, hiring and retaining replacements for departing employees and may lose significant expertise and talent, and our ability to realize the anticipated benefits of the Acquisition may be materially and adversely affected. No assurance can be given that we will be able to attract or retain key employees to the same extent that Avalon or Sen Lang has been able to attract or retain employees in the past.
Avalon and Sen Lang will incur significant transaction and Acquisition-related transition costs in connection with the Acquisition.
Avalon and Sen Lang expect that they will incur significant, non-recurring costs in connection with consummating the Acquisition and integrating the operations of the two companies post-closing. Avalon and/or Sen Lang may incur additional costs to retain key employees. Avalon and/or Sen Lang will also incur significant fees and expenses relating to financing arrangements and legal services (including any costs that would be incurred in defending against any potential class action lawsuits and derivative lawsuits in connection with the Acquisition if any such proceedings are brought), accounting and other fees and costs, associated with consummating the Acquisition. Some of these costs are payable regardless of whether the Acquisition is completed. Though Avalon and Sen Lang continue to assess the magnitude of these costs, additional unanticipated costs may be incurred in the Acquisition and the integration of the businesses of Avalon and Sen Lang.
The unaudited pro forma financial information included in this proxy statement is preliminary and our actual financial position or results of operations after the Acquisition may differ materially.
The unaudited pro forma financial information in this proxy statement is presented for illustrative purposes only and is not necessarily indicative of what our actual financial position or results of operations would have been had the Acquisition been completed on the dates indicated. The unaudited pro forma financial information reflects adjustments, which are based upon estimates, to allocate the purchase price to tangible and identifiable intangible assets acquired and liabilities assumed, based on their estimated acquisition-date fair values. The purchase price allocation reflected in this document is preliminary, and a final determination of the fair value of assets acquired and liabilities assumed will be based on the actual net tangible and intangible assets and liabilities of Sen Lang that existed as of the date of the completion of the Acquisition. Accordingly, the final purchase accounting adjustments may differ materially from the pro forma information reflected in this proxy statement. For more information, please see the section entitled “Unaudited Pro Forma Condensed Combined Financial Information.”
Avalon may be the target of securities class action and derivative lawsuits which could result in substantial costs and may delay or prevent the Acquisition from being completed.
Securities class action lawsuits and derivative lawsuits are often brought against public companies that have entered into acquisition agreements. Even if the lawsuits are without merit, defending against these claims can result in substantial costs and divert management time and resources. An adverse judgment could result in monetary damages, which could have a negative impact on Avalon’s liquidity and financial condition. Additionally, if a plaintiff is successful in obtaining an injunction prohibiting completion of the Acquisition, then that injunction may delay or prevent the Acquisition from being completed, which may adversely affect Avalon’s business, financial position and results of operations. As of the date of this proxy statement, no such lawsuits have been filed in connection with the Acquisition and we cannot predict whether any will be filed.
32
The lack of a public market for Sen Lang shares makes it difficult to determine the fair market value of the Sen Lang shares, and Avalon may pay more than the fair market value of the Sen Lang shares.
Sen Lang is privately held and its share capital is not traded in any public market. The lack of a public market makes it extremely difficult to determine Sen Lang’s fair market value. Because the percentage of Avalon’s equity to be issued to the Sen Lang Shareholders was determined based on negotiations between the parties, it is possible that Avalon may pay more than the aggregate fair market value for Sen Lang.
Additional Risks Relating to the Post-Acquisition Company after Completion of the Acquisition
The post-Acquisition company will be subject to the risks that each of Avalon and Sen Lang faces.
Following completion of the Acquisition, the post-Acquisition company will be subject to numerous risks and uncertainties, including the risks faced by each of Avalon and Sen Lang, which are described in the sections of this proxy statement entitled “Risk Factors—Risks Related to SenlangBio” and entitled “Risk Factors—Risks Related to Avalon.” If any such risks actually occur, the business, financial condition, results of operations or cash flows of the post-Acquisition company could be materially adversely affected.
The market price for shares of Avalon Common Stock may be affected by factors different from those affecting the market price for Sen Lang Shares.
Upon completion of the Acquisition, holders of Sen Lang Shares will become holders of Avalon Common Stock. Avalon’s and Sen Lang’s respective businesses differ, and accordingly the results of operations of the post-Acquisition company, and the market price of the common stock of the post-Acquisition company, will be affected by factors different from those currently affecting the results of operations of Avalon and Sen Lang. For a discussion of the businesses of Avalon and Sen Lang and of certain factors to consider in connection with those businesses, please see the sections entitled “Information About Sen Lang,” “Information About Avalon,” “Risk Factors—Risks Related to SenlangBio” and “Risk Factors—Risks Related to Avalon.”
The market price for shares of Avalon Common Stock may decline as a result of the Acquisition, including as a result of some Avalon stockholders adjusting their portfolios.
The market value of Avalon Common Stock at the time of consummation of the Acquisition may vary significantly from the price of Avalon Common Stock on the date the Purchase Agreement was executed, the date of this proxy statement and the date of the Avalon annual meeting. Following consummation of the Acquisition, the market price of Avalon Common Stock may decline if, among other things, the costs related to the Acquisition are greater than expected, Avalon does not achieve the perceived benefits of the Acquisition as rapidly or to the extent anticipated by financial or industry analysts or the effect of the Acquisition on Avalon’s financial position, results of operations or cash flows is not consistent with the expectations of financial or industry analysts.
In addition, sales of Avalon Common Stock by Avalon’s stockholders after the completion of the Acquisition may cause the market price of Avalon Common Stock to decrease. Based on the number of shares of Avalon Common Stock outstanding as of June 30, 2021, the latest practicable date before the date of this proxy statement, 166,080,919 shares of Avalon Common Stock are expected to be issued and outstanding immediately after the closing of the Acquisition (not taking into account any Exchange Shares that may be issued following the six month anniversary of the closing). Many Avalon stockholders and former Sen Lang Shareholders may decide not to hold the shares of Avalon Common Stock that they receive in the Acquisition. Other Avalon stockholders, such as funds with limitations on their permitted holdings of stock in individual issuers, may be required to sell the shares of Avalon Common Stock that they receive in the Acquisition. Such sales of Avalon Common Stock could have the effect of depressing the market price for Avalon Common Stock and may take place promptly following the closing of the Acquisition.
Any of these events may make it more difficult for Avalon to sell equity or equity-related securities, dilute your ownership interest in Avalon and have an adverse impact on the price of Avalon Common Stock.
33
Avalon does not expect to declare any cash dividends in the foreseeable future.
After the completion of the Acquisition, Avalon does not anticipate declaring any cash dividends to holders of Avalon Common Stock in the foreseeable future. Consequently, investors may need to rely on sales of their shares after price appreciation, which may never occur, as the only way to realize any future gains on their investment.
The Acquisition may not be accretive, and may be dilutive, to the post-Acquisition company’s earnings per share, which may negatively affect the market price of shares of Avalon Common Stock.
Avalon currently believes the Acquisition will result in a number of benefits, including (i) a diverse pipeline of cell therapy product candidates, (ii) expanded footprint in China, (iii) operational synergies and (iv) an experienced management team, and that the Acquisition will be accretive to the post-Acquisition company’s earnings. This belief is based, in part, on preliminary current estimates that may materially change. In addition, future events and conditions, including adverse changes in market conditions, additional transaction and integration-related costs and other factors such as the failure to realize some or all of the anticipated benefits of the Acquisition, could decrease or delay the accretion that is currently anticipated or could result in dilution. Any dilution of, or decrease in or delay of any accretion to, the post-Acquisition company’s earnings per share could cause the price of shares of Avalon Common Stock to decline or grow at a reduced rate.
An investment in Avalon Common Stock involves a high degree of risk. In determining whether to purchase Avalon Common Stock, an investor should carefully consider all of the material risks described below, together with the other information contained in this proxy statement before making a decision to purchase Avalon’s securities.
Avalon is, and will continue to be, subject to the risks described in Part I, Item 1A in Avalon’s Annual Report on Form 10-K for the year ended December 31, 2020, which Annual Report is being sent to stockholders together with this proxy statement.
34
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
On June 13, 2021, Avalon entered into a share purchase agreement (the “Purchase Agreement”) with Senlang. Pursuant to the Purchase Agreement, subject to the satisfaction of the conditions to closing, including approval by the Avalon stockholders pursuant to the rules of the Nasdaq Stock Market, Avalon will acquire all of the issued and outstanding securities of Senlang in consideration of 81,000,000 shares of Avalon common stock. The following unaudited pro forma consolidated combined financial statements present the historical consolidated financial statements of Avalon GloboCare Corp. and Subsidiaries (“Avalon”) and the historical consolidated financial statements of Lonlon Biotech Ltd. and Subsidiaries (“Senlang”), adjusted as if Avalon had acquired Senlang.
The unaudited pro forma consolidated combined balance sheet combines the historical consolidated balance sheet of Avalon and the historical consolidated balance sheet of Senlang as of March 31, 2021, giving effect to the acquisition as if they had been consummated on March 31, 2021. The unaudited pro forma consolidated combined statement of operations and comprehensive loss for the three months ended March 31, 2021 combines the historical consolidated statement of operations and comprehensive loss of Avalon and the historical consolidated statement of operations and comprehensive loss of Senlang, giving effect to the acquisition as if they had been consummated on January 1, 2020, the beginning of the earliest period presented. The unaudited pro forma consolidated combined statement of operations and comprehensive loss for the year ended December 31, 2020 combines the historical consolidated statement of operations and comprehensive loss of Avalon and the historical consolidated statement of operations and comprehensive loss of Senlang, giving effect to the acquisition as if they had been consummated on January 1, 2020, the beginning of the earliest period presented. The historical consolidated financial statements have been adjusted in the unaudited pro forma consolidated combined financial statements to give pro forma effect to events that are: (1) directly attributable to the acquisition; (2) factually supportable; and (3) with respect to the statement of operations, expected to have a continuing impact on Avalon’s results following the completion of the acquisition.
The unaudited pro forma consolidated combined financial statements have been developed from and should be read in conjunction with:
| ● | The accompanying notes to the unaudited pro forma consolidated combined financial statements; |
| ● | The historical consolidated financial statements and related notes of Avalon as of March 31, 2021, for the three months ended March 31, 2021 and for the year ended December 31, 2020, as well as “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” included in Avalon’s Quarterly Report on Form 10Q for the three months ended March 31, 2021 and Annual Report on Form 10-K for the year ended December 31, 2020, which were filed with the Securities and Exchange Commission; and |
| ● | The historical consolidated financial statements of Senlang as of March 31, 2021 and for the three months ended March 31, 2021 and for the year ended December 31, 2020, which are contained elsewhere in this Form 8-K/A. |
35
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
UNAUDITED PRO FORMA CONSOLIDATED COMBINED BALANCE SHEET
As of March 31, 2021
| Historical | Pro Forma | |||||||||||||||||||||||
| Avalon | Lonlon | |||||||||||||||||||||||
| GloboCare Corp. | Biotech Ltd. | Pro Forma Adjustments | Pro Forma | |||||||||||||||||||||
| and Subsidiaries | and Subsidiaries | Dr. | Cr. | Combined | ||||||||||||||||||||
| ASSETS | ||||||||||||||||||||||||
| CURRENT ASSETS: | ||||||||||||||||||||||||
| Cash | $ | 1,692,540 | $ | 188,887 | $ | - | $ | - | $ | 1,881,427 | ||||||||||||||
| Accounts receivable | - | 636,746 | - | - | 636,746 | |||||||||||||||||||
| Rent receivable | 23,471 | - | - | - | 23,471 | |||||||||||||||||||
| Deferred financing costs | 152,438 | - | - | - | 152,438 | |||||||||||||||||||
| Recoverable VAT | - | 343,755 | - | 343,755 | a | - | ||||||||||||||||||
| Inventory | - | 105,743 | - | 105,743 | a | - | ||||||||||||||||||
| Prepaid expenses and other current assets | 295,731 | 60,949 | 449,498 | a | - | 806,178 | ||||||||||||||||||
| Total Current Assets | 2,164,180 | 1,336,080 | 449,498 | 449,498 | 3,500,260 | |||||||||||||||||||
| NON-CURRENT ASSETS: | ||||||||||||||||||||||||
| Rent receivable - noncurrent portion | 109,174 | - | - | - | 109,174 | |||||||||||||||||||
| Security deposit | 19,662 | 49,843 | - | - | 69,505 | |||||||||||||||||||
| Deferred leasing costs | 133,359 | - | - | - | 133,359 | |||||||||||||||||||
| Operating lease right-of-use assets, net | 241,729 | 266,406 | - | - | 508,135 | |||||||||||||||||||
| Property and equipment, net | 439,962 | 2,268,076 | - | - | 2,708,038 | |||||||||||||||||||
| Investment in real estate, net | 7,644,950 | - | - | - | 7,644,950 | |||||||||||||||||||
| Equity method investment | 532,199 | - | - | - | 532,199 | |||||||||||||||||||
| Intangible assets | - | - | 97,556,672 | b | - | 97,556,672 | ||||||||||||||||||
| Total Non-current Assets | 9,121,035 | 2,584,325 | 97,556,672 | - | 109,262,032 | |||||||||||||||||||
| Total Assets | $ | 11,285,215 | $ | 3,920,405 | $ | 98,006,170 | $ | 449,498 | $ | 112,762,292 | ||||||||||||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||||||||||||||||||
| CURRENT LIABILITIES: | ||||||||||||||||||||||||
| Accrued professional fees | $ | 1,445,225 | $ | - | $ | - | $ | 650,000 | d | $ | 2,095,225 | |||||||||||||
| Accrued research and development fees | 432,500 | - | - | - | 432,500 | |||||||||||||||||||
| Accrued payroll liability and directors’ compensation | 165,846 | 102,329 | - | - | 268,175 | |||||||||||||||||||
| Accounts payable | - | 564,192 | 564,192 | e | - | - | ||||||||||||||||||
| Accrued leasehold improvements liabilities | - | 261,106 | 261,106 | e | - | - | ||||||||||||||||||
| Accrued liabilities and other payables | 344,453 | 62,938 | - | 1,175,995 | e | 1,583,386 | ||||||||||||||||||
| Notes payable | - | 1,373,480 | - | - | 1,373,480 | |||||||||||||||||||
| Accrued liabilities and other payables - related parties | 313,105 | - | - | - | 313,105 | |||||||||||||||||||
| Deferred revenue | - | 167,201 | 167,201 | e | - | - | ||||||||||||||||||
| Deferred grant income | - | 183,496 | 183,496 | e | - | - | ||||||||||||||||||
| Operating lease obligation | 132,657 | 151,167 | - | - | 283,824 | |||||||||||||||||||
| Note payable - related party | 390,000 | 244,174 | - | - | 634,174 | |||||||||||||||||||
| Total Current Liabilities | 3,223,786 | 3,110,083 | 1,175,995 | 1,825,995 | 6,983,869 | |||||||||||||||||||
| NON-CURRENT LIABILITIES: | ||||||||||||||||||||||||
| Deferred grant income - noncurrent portion | - | 304,617 | - | - | 304,617 | |||||||||||||||||||
| Operating lease obligation - noncurrent portion | 109,337 | 52,377 | - | - | 161,714 | |||||||||||||||||||
| Loan payable - related party | 3,305,249 | - | - | - | 3,305,249 | |||||||||||||||||||
| Total Non-current Liabilities | 3,414,586 | 356,994 | - | - | 3,771,580 | |||||||||||||||||||
| Total Liabilities | 6,638,372 | 3,467,077 | 1,175,995 | 1,825,995 | 10,755,449 | |||||||||||||||||||
| SHAREHOLDERS’ EQUITY: | ||||||||||||||||||||||||
| Preferred stock, $0.0001 par value; 10,000,000 shares authorized; no shares issued and outstanding | - | - | - | - | - | |||||||||||||||||||
| Common stock, $0.0001 par value; 490,000,000 shares authorized; 84,943,564 shares issued and 84,423,564 shares outstanding 165,943,564 pro forma shares issued and 165,423,564 pro forma shares outstanding | 8,494 | - | - | 8,100 | b | 16,594 | ||||||||||||||||||
| Additional paid-in capital | 49,755,996 | 8,946,197 | 8,946,197 | c | 98,001,900 | b | 147,757,896 | |||||||||||||||||
| Ordinary shares | - | 10,001 | 10,001 | c | - | - | ||||||||||||||||||
| Less: common stock held in treasury, at cost; 520,000 shares | (522,500 | ) | - | - | - | (522,500 | ) | |||||||||||||||||
| Accumulated deficit | (44,408,493 | ) | (8,515,294 | ) | 650,000 | d | 8,515,294 | c | (45,058,493 | ) | ||||||||||||||
| Statutory reserve | 6,578 | - | - | - | 6,578 | |||||||||||||||||||
| Accumulated other comprehensive (loss) income | (193,232 | ) | 12,424 | 12,424 | c | - | (193,232 | ) | ||||||||||||||||
| Total shareholders’ equity | 4,646,843 | 453,328 | 9,618,622 | 106,525,294 | 102,006,843 | |||||||||||||||||||
| Total Liabilities and Shareholders’ Equity | $ | 11,285,215 | $ | 3,920,405 | $ | 10,794,617 | $ | 108,351,289 | $ | 112,762,292 | ||||||||||||||
36
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
UNAUDITED PRO FORMA CONSOLIDATED COMBINED STATEMENT OF OPERATIONS AND COMPREHENSIVE LOSS
Three Months Ended March 31, 2021
| Historical | Pro Forma | |||||||||||||||||||||||
| Avalon | Lonlon | |||||||||||||||||||||||
| GloboCare Corp. | Biotech Ltd. | Pro Forma Adjustments | Pro Forma | |||||||||||||||||||||
| and Subsidiaries | and Subsidiaries | Dr. | Cr. | Combined | ||||||||||||||||||||
| REVENUES | ||||||||||||||||||||||||
| Real property rental | $ | 289,774 | $ | - | $ | - | $ | - | $ | 289,774 | ||||||||||||||
| General laboratory testing | - | 945,648 | - | - | 945,648 | |||||||||||||||||||
| Immunology and hematology testing | - | 301,857 | - | - | 301,857 | |||||||||||||||||||
| Total Revenues | 289,774 | 1,247,505 | - | - | 1,537,279 | |||||||||||||||||||
| COSTS AND EXPENSES | ||||||||||||||||||||||||
| Real property operating expenses | 216,894 | - | - | - | 216,894 | |||||||||||||||||||
| General laboratory testing | - | 347,911 | - | - | 347,911 | |||||||||||||||||||
| Immunology and hematology testing | - | 89,498 | - | - | 89,498 | |||||||||||||||||||
| Total Costs and Expenses | 216,894 | 437,409 | - | - | 654,303 | |||||||||||||||||||
| Real Property Opearting Income | 72,880 | - | - | - | 72,880 | |||||||||||||||||||
| Gross Profit from General Laboratory Testing | - | 597,737 | - | - | 597,737 | |||||||||||||||||||
| Gross Profit from Immunology and Hematology Testing | - | 212,359 | - | - | 212,359 | |||||||||||||||||||
| Total Gross Profit | 72,880 | 810,096 | - | - | 882,976 | |||||||||||||||||||
| OTHER OPERATING EXPENSES: | ||||||||||||||||||||||||
| Professional fees | 1,381,178 | - | 24,041 | a | - | 1,405,219 | ||||||||||||||||||
| Compensation and related benefits | 562,006 | - | 86,204 | a | - | 648,210 | ||||||||||||||||||
| Research and development expenses | 213,188 | 565,331 | - | - | 778,519 | |||||||||||||||||||
| General and administrative expenses | - | 406,188 | - | 406,188 | a | - | ||||||||||||||||||
| Other general and administrative expenses | 220,096 | - | 295,943 | a | - | 516,039 | ||||||||||||||||||
| Selling and marketing expenses | - | 52,707 | - | - | 52,707 | |||||||||||||||||||
| Amortization | - | - | 2,439,000 | c | - | 2,439,000 | ||||||||||||||||||
| Grant income | - | (123,467 | ) | - | - | (123,467 | ) | |||||||||||||||||
| Total Other Operating Expenses | 2,376,468 | 900,759 | 2,845,188 | 406,188 | 5,716,227 | |||||||||||||||||||
| LOSS FROM OPERATIONS | (2,303,588 | ) | (90,663 | ) | (2,845,188 | ) | (406,188 | ) | (4,833,251 | ) | ||||||||||||||
| OTHER (EXPENSE) INCOME | ||||||||||||||||||||||||
| Interest expense | - | (13,647 | ) | - | - | (13,647 | ) | |||||||||||||||||
| Interest expense - related party | (45,149 | ) | (2,683 | ) | - | - | (47,832 | ) | ||||||||||||||||
| Loss from equity method investment | (18,514 | ) | - | - | - | (18,514 | ) | |||||||||||||||||
| Other income | 133 | 226 | - | - | 359 | |||||||||||||||||||
| Total Other Expense, net | (63,530 | ) | (16,104 | ) | - | - | (79,634 | ) | ||||||||||||||||
| LOSS BEFORE INCOME TAXES | (2,367,118 | ) | (106,767 | ) | (2,845,188 | ) | (406,188 | ) | (4,912,885 | ) | ||||||||||||||
| INCOME TAXES | - | 28,513 | - | - | 28,513 | |||||||||||||||||||
| NET LOSS | $ | (2,367,118 | ) | $ | (135,280 | ) | $ | (2,845,188 | ) | $ | (406,188 | ) | $ | (4,941,398 | ) | |||||||||
| COMPREHENSIVE LOSS: | ||||||||||||||||||||||||
| NET LOSS | $ | (2,367,118 | ) | $ | (135,280 | ) | $ | (2,845,188 | ) | $ | (406,188 | ) | $ | (4,941,398 | ) | |||||||||
| OTHER COMPREHENSIVE LOSS | ||||||||||||||||||||||||
| Unrealized foreign currency translation loss | (2,722 | ) | (577 | ) | - | - | (3,299 | ) | ||||||||||||||||
| COMPREHENSIVE LOSS | $ | (2,369,840 | ) | $ | (135,857 | ) | $ | (2,845,188 | ) | $ | (406,188 | ) | $ | (4,944,697 | ) | |||||||||
| NET LOSS PER COMMON SHARE: | ||||||||||||||||||||||||
| Basic and diluted | $ | (0.03 | ) | $ | (0.03 | ) b | ||||||||||||||||||
| WEIGHTED AVERAGE COMMON SHARES OUTSTANDING: | ||||||||||||||||||||||||
| Basic and diluted | 83,413,154 | 164,413,154 | ||||||||||||||||||||||
37
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
UNAUDITED PRO FORMA CONSOLIDATED COMBINED STATEMENT OF OPERATIONS AND COMPREHENSIVE LOSS
Year Ended December 31, 2020
| Historical | Pro Forma | |||||||||||||||||||||||
| Avalon | Lonlon | |||||||||||||||||||||||
| GloboCare Corp. | Biotech Ltd. | Pro Forma Adjustments | Pro Forma | |||||||||||||||||||||
| and Subsidiaries | and Subsidiaries | Dr. | Cr. | Combined | ||||||||||||||||||||
| REVENUES | ||||||||||||||||||||||||
| Real property rental | $ | 1,206,854 | $ | - | $ | - | $ | - | $ | 1,206,854 | ||||||||||||||
| Medical related consulting services - related parties | 170,908 | - | - | - | 170,908 | |||||||||||||||||||
| General laboratory testing | - | 649,932 | - | - | 649,932 | |||||||||||||||||||
| Immunology and hematology testing | - | 440,183 | - | - | 440,183 | |||||||||||||||||||
| Total Revenues | 1,377,762 | 1,090,115 | - | - | 2,467,877 | |||||||||||||||||||
| COSTS AND EXPENSES | ||||||||||||||||||||||||
| Real property operating expenses | 851,754 | - | - | - | 851,754 | |||||||||||||||||||
| Medical related consulting services - related parties | 135,805 | - | - | - | 135,805 | |||||||||||||||||||
| General laboratory testing | - | 343,794 | - | - | 343,794 | |||||||||||||||||||
| Immunology and hematology testing | - | 197,444 | - | - | 197,444 | |||||||||||||||||||
| Total Costs and Expenses | 987,559 | 541,238 | - | - | 1,528,797 | |||||||||||||||||||
| Real Property Opearting Income | 355,100 | - | - | - | 355,100 | |||||||||||||||||||
| Gross Profit from Medical Related Consulting Services | 35,103 | - | - | - | 35,103 | |||||||||||||||||||
| Gross Profit from General Laboratory Testing | - | 306,138 | - | - | 306,138 | |||||||||||||||||||
| Gross Profit from Immunology and Hematology Testing | - | 242,739 | - | - | 242,739 | |||||||||||||||||||
| Total Gross Profit | 390,203 | 548,877 | - | - | 939,080 | |||||||||||||||||||
| OTHER OPERATING EXPENSES: | ||||||||||||||||||||||||
| Professional fees | 6,553,009 | - | 73,397 | a | - | 6,626,406 | ||||||||||||||||||
| Compensation and related benefits | 4,156,150 | - | 292,439 | a | - | 4,448,589 | ||||||||||||||||||
| Research and development expenses | 883,855 | 2,813,250 | - | - | 3,697,105 | |||||||||||||||||||
| General and administrative expenses | - | 925,438 | - | 925,438 | a | - | ||||||||||||||||||
| Other general and administrative expenses | 1,251,208 | - | 559,602 | a | - | 1,810,810 | ||||||||||||||||||
| Selling and marketing expenses | - | 163,145 | - | - | 163,145 | |||||||||||||||||||
| Amortization | - | - | 9,756,000 | c | - | 9,756,000 | ||||||||||||||||||
| Grant income | - | (929,505 | ) | - | - | (929,505 | ) | |||||||||||||||||
| Total Other Operating Expenses | 12,844,222 | 2,972,328 | 10,681,438 | 925,438 | 25,572,550 | |||||||||||||||||||
| LOSS FROM OPERATIONS | (12,454,019 | ) | (2,423,451 | ) | (10,681,438 | ) | (925,438 | ) | (24,633,470 | ) | ||||||||||||||
| OTHER (EXPENSE) INCOME | ||||||||||||||||||||||||
| Interest expense | - | (12,397 | ) | - | - | (12,397 | ) | |||||||||||||||||
| Interest expense - related party | (168,762 | ) | (11,169 | ) | - | - | (179,931 | ) | ||||||||||||||||
| Loss from equity method investment | (51,673 | ) | - | - | - | (51,673 | ) | |||||||||||||||||
| Other (expense) income | (4,984 | ) | 4,654 | - | - | (330 | ) | |||||||||||||||||
| Total Other Expense, net | (225,419 | ) | (18,912 | ) | - | - | (244,331 | ) | ||||||||||||||||
| LOSS BEFORE INCOME TAXES | (12,679,438 | ) | (2,442,363 | ) | (10,681,438 | ) | (925,438 | ) | (24,877,801 | ) | ||||||||||||||
| INCOME TAXES | - | - | - | - | - | |||||||||||||||||||
| NET LOSS | $ | (12,679,438 | ) | $ | (2,442,363 | ) | $ | (10,681,438 | ) | $ | (925,438 | ) | $ | (24,877,801 | ) | |||||||||
| COMPREHENSIVE LOSS: | ||||||||||||||||||||||||
| NET LOSS | $ | (12,679,438 | ) | $ | (2,442,363 | ) | $ | (10,681,438 | ) | $ | (925,438 | ) | $ | (24,877,801 | ) | |||||||||
| OTHER COMPREHENSIVE INCOME | ||||||||||||||||||||||||
| Unrealized foreign currency translation gain | 67,237 | 58,807 | - | - | 126,044 | |||||||||||||||||||
| COMPREHENSIVE LOSS | $ | (12,612,201 | ) | $ | (2,383,556 | ) | $ | (10,681,438 | ) | $ | (925,438 | ) | $ | (24,751,757 | ) | |||||||||
| NET LOSS PER COMMON SHARE: | ||||||||||||||||||||||||
| Basic and diluted | $ | (0.16 | ) | $ | (0.15 | ) b | ||||||||||||||||||
| WEIGHTED AVERAGE COMMON SHARES OUTSTANDING: | ||||||||||||||||||||||||
| Basic and diluted | 79,508,149 | 160,508,149 | ||||||||||||||||||||||
38
[1] Basis of Pro Forma Presentation
The unaudited pro forma consolidated combined financial statements have been prepared assuming the acquisition is accounted for as a business combination using the acquisition method of accounting under Financial Accounting Standards Board (“FASB”) ASC 805, Business Combinations (“ASC 805”). For business combinations under ASC 805, acquisition-related transaction costs are not included as a component of consideration transferred but are accounted for as expenses in the periods in which such costs are incurred. Acquisition-related transaction costs include advisory, legal, accounting fee and others.
The unaudited pro forma consolidated combined financial statements reflect adjustments, based on available information and certain assumptions that Avalon believes are reasonable, attributable to the following:
| ● | The acquisition of Senlang, which will be accounted for as a business combination, with Avalon identified as the acquirer, and the issuance of shares of Avalon common stock as acquisition consideration. Avalon is considered the accounting acquirer since immediately following the closing: (i) Avalon stockholders will own a majority of the voting rights of the combined company; (ii) Avalon will designate a majority (nine of ten) of the initial members of the board of directors of the combined company; (iii) Avalon’s senior management will hold the majority of the key positions in senior management of the combined company; and (iv) Avalon will continue to maintain its corporate headquarters in Freehold, New Jersey, United States. SenlangBio will continue to maintain operations in the Shijiazhuang High-tech Development Zone, Hebei Province, China; |
| ● | Adjustments to conform the classification of certain assets and liabilities in Senlang’s historical consolidated balance sheet to Avalon’s classification for similar assets and liabilities; |
| ● | Adjustments to conform the classification of expenses in Senlang’s historical consolidated statement of operations and comprehensive loss to Avalon’s classification for similar expenses; and |
| ● | The incurrence of acquisition-related expenses. |
The pro forma adjustments represent management’s estimates based on information available as of the date of this filing and are subject to change as additional information becomes available and additional analyses are performed. The pro forma financial statements are provided for illustrative purposes only and are not intended to represent what Avalon’s financial position or results of operations would have been had the acquisition actually been consummated on the assumed dates nor do they purport to project the future operating results or financial position of Avalon following the acquisition. The pro forma financial statements do not reflect future events that may occur after the acquisition, including, but not limited to, the anticipated realization of ongoing savings from potential operating efficiencies, cost savings, or economies of scale that Avalon may achieve with respect to the combined operations. Specifically, the pro forma statements of operations do not include the synergies expected to be achieved as a result of the acquisition and any associated costs that may be incurred to achieve the identified synergies. Additionally, Avalon cannot assure that additional charges will not be incurred in excess of those included in the pro forma additional legal, accounting, and advisory fees of $650,000 related to the acquisition, Avalon’s efforts to achieve operational synergies, or that management will be successful in its efforts to integrate the operations. The pro forma statement of operations also excludes the effects of costs associated with any restructuring and integration activities that may result from the acquisition. Further, the pro forma financial statements do not reflect the effect of any regulatory actions that may impact the results of Avalon following the acquisition.
Assumptions and estimates underlying the pro forma adjustments are described in the accompanying notes, which should be read in conjunction with the unaudited pro forma consolidated combined financial statements. In Avalon’s opinion, all adjustments that are necessary to present fairly the pro forma information have been made. The historical financial statements have been adjusted in the unaudited pro forma consolidated combined financial statements to give effect to the acquisition. These adjustments are directly attributable to the acquisition, factually supportable and, with respect to the unaudited pro forma consolidated combined statements of operations, expected to have a continuing impact on Avalon following the acquisition.
39
[2] Pro Forma Adjustments and Assumptions
Pro Forma Adjustments to the Consolidated Balance Sheet at March 31, 2021:
| a. | Represents the reclassification of recoverable VAT and inventory into prepaid expenses and other current assets. |
| b. | Reflects the issuance of 81,000,000 shares of Avalon common stock at a price of $1.21 per share (which was the market price of the Avalon common stock as of the date of the Purchase Agreement under the rules of the Nasdaq Stock Market) as consideration for acquisition of Senlang and adjustments to state Senlang’s assets acquired and liabilities assumed at fair value. A summary of the consideration paid and the preliminary fair value of the assets acquired and liabilities assumed is as follows: |
| Preliminary consideration: | ||||
| Avalon common stock issued to Senlang shareholders | 81,000,000 | |||
| Issued price | $ | 1.21 | ||
| Total consideration | $ | 98,010,000 | ||
| Preliminary fair value of assets acquired: | ||||
| Current assets | ||||
| Cash | $ | 188,887 | ||
| Accounts receivable | 636,746 | |||
| Recoverable VAT | 343,755 | |||
| Inventory | 105,743 | |||
| Other current assets | 60,949 | |||
| Non-current assets | ||||
| Security deposit | 49,843 | |||
| Operating lease right-of-use assets, net | 266,406 | |||
| Property and equipment, net | 2,268,076 | |||
| Intangible assets | 97,556,672 | |||
| Total preliminary fair value of assets acquired | $ | 101,477,077 | ||
| Preliminary fair value of liabilities assumed: | ||||
| Current liabilities | ||||
| Notes payable | $ | (1,373,480 | ) | |
| Notes payable - related party | (244,174 | ) | ||
| Accounts payable | (564,192 | ) | ||
| Salary payable | (102,329 | ) | ||
| Accrued leasehold improvements liabilities | (261,106 | ) | ||
| Accrued liabilities and other payables | (62,938 | ) | ||
| Deferred revenue | (167,201 | ) | ||
| Deferred grant income | (183,496 | ) | ||
| Operating lease obligation | (151,167 | ) | ||
| Non-current liabilities | ||||
| Deferred grant income - noncurrent portion | (304,617 | ) | ||
| Operating lease obligation - noncurrent portion | (52,377 | ) | ||
| Total preliminary fair value of liabilities assumed | $ | (3,467,077 | ) | |
| Net Assets acquired and liabilities assumed | $ | 98,010,000 | ||
40
The acquisition consideration is allocated to the acquired assets and assumed liabilities based on their estimated fair values, and any excess is initially allocated to identifiable intangible assets mainly consisting of cell and gene engineering technologies with the ability to generate innovative and transformative cellular immunotherapies for solid and hematologic cancers. The purchase price exceeded the fair value of net assets acquired by $97,556,672. The Company allocated the $97,556,672 excess to intangible assets, which will be amortized over 10 years. The initial allocation is subject to change upon the final valuation which is to be done at the time of closing. Such change could have a material impact on the Company’s financial statements.
| c. | Represents the elimination of Senlang’s historical equity balances. |
| d. | Represents the accrual of $650,000 in estimated legal, accounting, and advisory fees that are payable as a result of the acquisition of Senlang, which were not reflected in either Avalon’s or Senlang’s historical financial statements. |
| e. | Represents the reclassification of accounts payable, accrued leasehold improvements liabilities, deferred revenue, deferred grant income into accrued liabilities and other payables. |
Pro Forma Adjustments to the Consolidated Statement of Operations and Comprehensive Loss for the Three Months Ended March 31, 2021:
| a. | Represents a reclassification of general and administrative expenses into professional fees, compensation and related benefits, and other general and administrative expenses. |
| b. | The pro forma basic and diluted net loss per common share was computed by dividing pro forma net loss attributable to Avalon by the historical weighted average number of shares of common stock outstanding after giving effect to the issuance of 81,000,000 shares of Avalon common stock in connection with the acquisition of Senlang, as if the issuance had been completed on January 1, 2020. |
| c. | Represents amortization of intangible assets acquired from this acquisition. |
Pro Forma Adjustments to the Consolidated Statement of Operations and Comprehensive Loss for the Year Ended December 31, 2020:
| a. | Represents a reclassification of general and administrative expenses into professional fees, compensation and related benefits, and other general and administrative expenses. |
| b. | The pro forma basic and diluted net loss per common share was computed by dividing pro forma net loss attributable to Avalon by the historical weighted average number of shares of common stock outstanding after giving effect to the issuance of 81,000,000 shares of Avalon common stock in connection with the acquisition of Senlang, as if the issuance had been completed on January 1, 2020. |
| c. | Represents amortization of intangible assets acquired from this acquisition. |
41
[3] Unaudited Pro Forma Adjustment Reflects the Following Two Transactions:
Transaction 1:
| Intangible assets | 97,556,672 | |||||||
| Paid-in capital | 8,956,198 | |||||||
| Accumulated other comprehensive income | 12,424 | |||||||
| Accumulated deficit | 8,515,294 | |||||||
| Common stock | 8,100 | |||||||
| Additional paid-in capital | 98,001,900 |
The transaction reflects (i) the elimination of Senlang’s historical equity balances; (ii) the issuance of 81,000,000 shares of Avalon common stock at a price of $1.21 per share as consideration for acquisition of Senlang; (iii) and acquisition consideration exceeded the fair value of net assets acquired by $97,556,672, which the Company allocated to intangible assets mainly consisting of cell and gene engineering technologies with the ability to generate innovative and transformative cellular immunotherapies for solid and hematologic cancers.
Transaction 2:
| Accumulated deficit | 650,000 | |||||||
| Accrued professional fees | 650,000 |
To accrue $650,000 estimated additional legal, accounting, and advisory fees that are payable as a result of the acquisition of Senlang, which were not reflected in either Avalon’s or Senlang’s historical financial statements.
42
The Background of the Acquisition
The terms of the Purchase Agreement are the result of arm’s-length negotiations between representatives of Avalon and Sen Lang. The following is a brief discussion of the background of these negotiations. The following chronology does not purport to catalogue every conversation among Avalon, Sen Lang, and their respective representatives.
In the ordinary course of its business, Avalon’s board of directors, with the assistance of its senior management and advisors, regularly reviews the near-term and long-term strategy, performance, positioning, and operating prospects of Avalon with a view toward enhancing stockholder value. These reviews have included, from time to time, potential capital markets transactions and acquisitions, as well as a review of the potential benefits and risks associated with each such course of action.
From September 2020 through May 2021, Avalon and its advisors conducted due diligence on Sen Lang (which consists of a group of companies including the operating company SenlangBio, and the BVI company Sen Lang. For simplicity, all Sen Lang group entities are referred to as “Sen Lang”). A financial adviser conducted financial due diligence and delivered its final Financial Due Diligence Report on December 12, 2020. Lowenstein Sandler LLP, counsel to Avalon (“Lowenstein”) conducted limited U.S. intellectual property due diligence and delivered its IP Diligence Summary on April 8, 2021. JunHe, PRC counsel to Avalon (“JunHe”) conducted full legal due diligence and delivered an initial draft of its comprehensive due diligence report on April 12, 2021.
On April 19, 2021, Avalon’s board of directors held a telephonic meeting, with representatives from Lowenstein Sandler in attendance, and discussed the terms of the proposed acquisition of Sen Lang.
On April 27, 2021, Lowenstein Sandler provided an initial draft of the Purchase Agreement to Avalon, which Avalon provided to Sen Lang.
During the months of April and May 2021, Avalon continued its financial, regulatory, intellectual property and business-related review of Sen Lang. JunHe delivered its final due diligence report on May 17, 2021.
On May 24, 2021, Sen Lang delivered to Avalon a revised draft of the Purchase Agreement. On May 25, 2021, Lowenstein Sandler provided a further revised draft of the Purchase Agreement to Avalon, which Avalon provided to Sen Lang.
On June 1, 2021, Sen Lang delivered to Avalon an initial draft of the Sen Lang Disclosure Schedule to the Purchase Agreement. Avalon and JunHe reviewed and provided comments to the Disclosure Schedule on or about June 4, 2021. Sen Lang delivered a revised draft of the Disclosure Schedule on June 10, 2021, and after receiving further clarifying comments from JunHe, delivered the substantively final draft of the Disclosure Schedule on June 12, 2021.
On June 3, 2021, Sen Lang delivered to Avalon a further revised draft of the Purchase Agreement. Lowenstein Sandler delivered the substantively final draft of the Purchase Agreement to Avalon on June 7, 2021, which Avalon provided to Sen Lang.
During April through June, management of Avalon held various calls with members of the Board of Avalon to discuss the transactions related to the Purchase Agreement and the Equity Financing and responded to questions from various members of the Board.
In connection with the Purchase Agreement, Avalon and Sen Lang also negotiated several ancillary documents. Simultaneous to their entrance into the Purchase Agreement, Avalon and Sen Lang also entered into a Securities Exchange Agreement (“SEA”) and a Capital Increase Agreement (“CIA”) with Yueyin Datong (Tianjin) Asset Management Co. Ltd. (“Yueyin”), pursuant to which Yueyin committed to invest RMB 200,000,000 in SenlangBio in three installments in exchange for its right to convert its investment in SenlangBio, at its option, to Avalon shares or Sen Lang (BVI) shares. Yueyin is an affiliate of IN Capital, a healthcare PE fund active in China. In or about early March 2021, while Avalon was doing due diligence on Sen Lang, Avalon was approached by IN Capital for its interest in investing in SenlangBio simultaneously with Avalon’s acquisition of Sen Lang. Although a draft term sheet was circulated by Avalon as early as mid-March 2021 to IN Capital and after rounds of discussions as to the feasibility of the proposed structure of the investment, the term sheet was never signed. On or about May 12, 2021, Avalon delivered its first draft of SEA and CIA to IN Capital.
43
On May 25, 2021, Avalon received from IN Capital’s counsel its comments on the SEA and CIA, and held various calls initially with JunHe and Lowenstein, and then with In Capital’s counsel. After intensive discussion and several rounds of revisions in the next week or so with negotiations focusing on mainly the process and formula of securities conversion, a comprehensive draft was sent to IN Capital on or around June 3, 2021. On June 7, 2021, Avalon received comments from IN Capital that it would like to split its investments in SenlangBio into three installments. After this major change and other revisions, the final versions of the SEA and CIA were executed on June 13, 2021.
Avalon’s board of directors approved the Purchase Agreement, Securities Exchange Agreement and transactions contemplated thereby on June 13, 2021.
On June 24, 2021, the parties signed an Amendment 1 to both the SEA and CIA, to amend certain conversion formulas which would result in a small adjustment to the Investor’s equity percentage in Sen Lang if they exercise their options to convert to Sen Lang shares.
The Avalon board of directors unanimously (i) determined that the terms and provisions of the Purchase Agreement and the transactions contemplated thereby, including the Acquisition, are fair to, advisable and in the best interests of the Company and its stockholders, (ii) approved the Purchase Agreement and the transactions contemplated thereby, including the Acquisition, (iii) authorized, empowered and directed the Company to perform all of its obligations under the Purchase Agreement and related documents, and (iv) resolved to recommend the adoption of the Purchase Agreement by the stockholders of the Company in compliance with the rules of Nasdaq.
In the course of its evaluation of the Acquisition Agreement with Sen Lang, the Avalon Board held numerous meetings, consulted with Avalon’s senior executive management, Avalon’s outside legal counsel and advisors, and reviewed and assessed a significant amount of information, and considered a number of factors, including the following:
| ● | the Avalon Board’s belief that the acquisition of SenlangBio will significantly enhance Avalon’s capabilities and competitiveness in the cell and gene therapy sector. By adding 15 of SenlangBio’s autologous and universal (“off-the-shelf”) cell therapy candidates, it is expected to enriched Avalon’s R&D and therapeutic portfolio with potential applications to a wide array of hematologic malignancies and solid tumors. The Avalon’s Board believes this transformative acquisition will significantly contribute to sustainable and successful growth and development of Avalon and its business; |
| ● | the Avalon Board’s belief that the acquisition of SenlangBio will result in seamless vertical integration of upstream scientific and technology-based expertise, mid-stream bio-processing and bio-manufacturing capabilities, as well as downstream clinical components in the execution of clinical studies and caring of patients in cell therapy settings. The Avalon Board believes this will enhance Avalon’s ability to offer customers a fully-integrated, end-to-end platform, de-risking and accelerating Avalon’s discovery and development of pre-clinical and clinical programs; |
| ● | the Avalon Board’s consideration that acquisition of SenlangBio will include experienced members from the senior management team of SenlangBio who have expertise in discovery, research and clinical development in the sector of cell and gene therapy, which will be highly valued by potential pharmaceutical and biotechnology partners and will enhance the composition of the Avalon management team; |
| ● | the Avalon Board’s belief that the acquisition of SenlangBio will position Avalon to create partnership engagements with pharmaceutical and biotechnology companies that will yield significant revenue opportunities, based on the technical capabilities and robust assets of cell therapy candidates to be acquired from SenlangBio; |
44
| ● | the Avalon Board’s belief that the added scientific and clinical capabilities and merits from the acquisition of SenlangBio will provide the best opportunity to improve upon Avalon’s cash position by attracting investments, as well as driving significant value for shareholders in the years ahead; |
| ● | the historical market prices, volatility and trading information of Avalon Common Stock; |
| ● | the fact that the amount of Acquisition Shares was achieved through a series of arms’ length negotiations between the parties; |
| ● | the recommendation of Avalon’s management in favor of the Acquisition; |
| ● | the terms and conditions of the Acquisition Agreement, including the commitments by Avalon and Sen Lang to complete the Acquisition and the transactions contemplated thereby; |
| ● | the Avalon Board’s belief that, while the consummation of the Acquisition is subject to various regulatory approvals, such approvals were likely to be obtained without a material adverse impact on the respective businesses of Avalon and SenlangBio; and |
| ● | the terms of the proposed Equity Financing related to the Acquisition. |
The Avalon board of directors considered these advantages and opportunities against a number of other factors identified in its deliberations weighing negatively against the Acquisition, including:
| ● | the difficulties of combining the businesses of Avalon and SenlangBio based on, among other things, the different geographical locations and complexities of the two companies, including issues unique to the PRC, and potential disruption associated with the transactions and integrating the companies; | |
| ● | the challenges inherent in the management and operation of the post-Acquisition company, including the risk that integration costs may be greater than anticipated and may require greater than anticipated management attention and focus post-closing; | |
| ● | the risks and costs to Avalon in connection with the Acquisition (including if the Acquisition is not completed), either during the pendency of the Acquisition or following the closing, including the risks and costs associated with the potential diversion of management and employee attention, potential employee attrition and the potential effect on business, operations and financial results; | |
| ● | the potential that the fixed nature of the Acquisition Shares could result in Avalon delivering greater value to the Sen Lang Owners than had been anticipated by Avalon should the value of shares of Avalon Common Stock increase disproportionately from the date of the Acquisition Agreement; | |
| ● | the possibility that the anticipated benefits of the Acquisition may not be realized, recognizing the many challenges associated with successfully integrating the businesses of Avalon and SenlangBio, including the risk of not capturing all of the anticipated cost savings, synergies and operational efficiencies; | |
| ● | the fact that, assuming the consummation of the Acquisition, the dilution to Avalon stockholders as stockholders of the post-Acquisition company due to the issuance of all of the securities to be issued in connection with the Acquisition and the Equity Financing; | |
| ● | the fact that executive officers and directors of Avalon have interests in the Acquisition that may be different from, or in addition to, the interests of Avalon stockholders (please see the section entitled “The Acquisition—Interests of Avalon’s Directors and Officers in the Acquisition”); and | |
| ● | various other risks associated with the Acquisition and the businesses of Avalon, SenlangBio and the post-Acquisition company described in the section entitled “Risk Factors.” |
45
The foregoing discussion of the factors considered by the Avalon Board is not intended to be exhaustive, but rather includes the principal factors considered by the Avalon Board in reaching its conclusion and recommendation in relation to the Acquisition and the Avalon proposals included herein. In view of the wide variety of factors considered in connection with its evaluation of the Acquisition and the complexity of these matters, the Avalon Board did not find it useful and did not attempt to quantify or assign any relative or specific weights to the various factors that it considered in reaching its determination to approve the Acquisition Agreement and to make its recommendations to Avalon’s stockholders. In addition, individual members of the Avalon Board may have given differing weights to different factors. The Avalon board of directors conducted an overall review of the factors described above, including thorough discussions with Avalon’s management and outside legal and financial advisors. In considering the recommendation of the Avalon Board, Avalon’s stockholders should be aware that Avalon’s directors may have interests in the Acquisition that are different from, or in addition to, those of the Avalon stockholders generally. Please see the section entitled “Interests of Avalon Directors and Executive Officers in the Acquisition.”
Neither Sen Lang nor any of its subsidiaries owns any equity interest in SenlangBio. Instead, it controls and receives the economic benefits of SenlangBio’s business operation through a series of contractual arrangements.
On April 26, 2021, the PRC Subsidiary, which is indirectly wholly-owned by Sen Lang, entered into a series of contractual arrangements, or VIE Agreements, with SenlangBio and the 13 equity holders of SenlangBio, through which Sen Lang obtained control and became the primary beneficiary of SenlangBio, hereinafter referred to as the Reorganization. As a result, SenlangBio became Sen Lang’s “VIE.”
Sen Lang was established as an indirect holding company of the PRC Subsidiary.
46
The following chart illustrates Sen Lang’s corporate structure, including its subsidiaries, consolidated variable interest entity and VIE’s subsidiary as of June 24, 2021 (the issuance date of Sen Lang’s historical financial statements included herein):

VIE Agreements with SenlangBio
Upon the completion of the Reorganization, Sen Lang, through the PRC Subsidiary, entered into the following contractual arrangements with the VIE and the VIE’s 13 shareholders that enabled Sen Lang to (1) have power to direct the activities that most significantly affects the economic performance of the VIE, and (2) receive the economic benefits of the VIE that could be significant to the VIE. Accordingly, the PRC Subsidiary was considered the primary beneficiary of the VIE and had consolidated the VIE and the VIE’s subsidiary’s financial results of operations, assets and liabilities in Sen Lang’s consolidated financial statements.
Contracts that give Sen Lang effective control of the VIE
Equity Pledge Agreement
Under the Equity Pledge Agreement between the PRC Subsidiary, SenlangBio and SenlangBio’s shareholders, SenlangBio’s shareholders agree to pledge all of their equity interests in SenlangBio to the PRC Subsidiary to guarantee the performance of SenlangBio and SenlangBio’s shareholders’ obligations under the Exclusive Technical Consultation and Service Agreement, the Exclusive Purchase Option Agreement, the Shareholder’s Rights Proxy Agreement, and the Spousal Consent (“Transaction Agreements”). Under the terms of the Equity Pledge Agreement, in the event that SenlangBio or SenlangBio’s shareholders breach their respective contractual obligations under the Transaction Agreements or the Equity Pledge Agreement, the PRC Subsidiary, as pledgee, is entitled to directly exercise the pledge right and to notify SenlangBio’s shareholders to immediately repay or pay the loans or other payables under the Transaction Agreements. SenlangBio’s shareholders further agreed not to dispose of the pledged equity interests without prior written consent from the PRC Subsidiary.
47
The pledge is effective on the date when the registration of the equity pledge is completed with the competent administration for industry and commerce, and the term of validity of the pledge is the same as the longest term of validity in the Transaction Agreements.
The purposes of the Equity Pledge Agreement are to (1) guarantee the performance of SenlangBio and SenlangBio’s shareholders’ obligations under the Transaction Agreements, (2) make sure SenlangBio’s shareholders do not transfer or assign the pledged equity interests, or create or allow any encumbrance that would prejudice interests of the PRC Subsidiary without prior written consent from the PRC Subsidiary, and (3) provide the PRC Subsidiary control over SenlangBio.
In the event SenlangBio or SenlangBio’s shareholders breaches their contractual obligations under the Transaction Agreements or Equity Pledge Agreement, the PRC Subsidiary will be entitled to (1) be compensated on a preferential basis with the proceeds from the conversion, auction or sale of the pledged equity, and (2) notify SenlangBio’s shareholders to immediately repay the loans or other payables under the Transaction Agreements.
Exclusive Purchase Option Agreement
Under the Exclusive Purchase Option Agreement, SenlangBio’s shareholders irrevocably grants the PRC Subsidiary (or its designee) an exclusive right to purchase the equity held by SenlangBio’s shareholders in the SenlangBio in whole or in part at any time during the term of the agreement; SenlangBio also irrevocably grants the PRC Subsidiary (or its designee) an exclusive right to purchase the assets owned by SenlangBio in whole or in part at any time during the term of the agreement.
With respect to consideration for equity purchase, the PRC Subsidiary has the right to purchase all or part of the equity held by SenlangBio’s shareholders in SenlangBio at the lowest price permitted by the PRC laws. With respect to the price for asset purchase, the PRC Subsidiary has the right to purchase SenlangBio’s assets at a price equivalent of the net book value of the purchased assets; provided that if the minimum price permitted by the PRC law is higher than the net book value, the minimum price permitted by the PRC laws will prevail.
Under the Exclusive Purchase Option Agreement, the PRC Subsidiary may purchase all or part of the equity held by SenlangBio’s shareholders in SenlangBio and all or part of the assets owned by SenlangBio at any time to the extent permitted by PRC laws. The Exclusive Purchase Option Agreement, together with the Equity Pledge Agreement, Exclusive Technical Consultation and Service Agreement, and the Proxy Agreement, enable the PRC Subsidiary to exercise effective control over SenlangBio. The Exclusive Purchase Option Agreement remains in effect, unless terminated by the PRC Subsidiary by giving a thirty (30) day advance written notice.
Shareholder’s Rights Proxy Agreement
Under the Shareholder’s Rights Proxy Agreement, SenlangBio’s shareholders authorize any entity or individual designated by the PRC Subsidiary to act on their behalf as their exclusive agent to exercise all shareholder’s rights under the PRC laws and SenlangBio’s Articles of Association, including but not limited to: (a) to convene, attend and vote on all the matters during shareholders’ meetings; (b) to transfer, pledge or dispose of, or create encumbrance on the equity; (c) to receive dividends; (d) to participate in judicial proceedings or execute legal documents in relation to shareholders’ rights; (e) to appoint legal representatives, directors and officers of SenlangBio; and (f) to enter into contracts and exercise the Exclusive Purchase Option Agreement.
The Shareholder’s Rights Proxy Agreement shall remain effective until the earlier of: (a) the date on which SenlangBio’s shareholders are no longer the nominee or actual shareholders of the PRC Subsidiary; (b) the date on which the PRC Subsidiary requests the proxy to be terminated in writing; or (c) the date on which the assets and licenses of SenlangBio have been fully transferred to the PRC Subsidiary.
48
Contracts that enable Sen Lang to receive substantially all of the economic benefits from the VIE
Exclusive Technical Consultation and Service Agreement
Pursuant to the Exclusive Technical Consultation and Service Agreement between the PRC Subsidiary and SenlangBio, the PRC Subsidiary provides SenlangBio with technical consultation and services, including conducting market research, assisting with developing management and sales plans, implementing relevant technology application, and providing other consultation services for computer network, finance, business, legal affairs, operation, human resources, and other aspects of SenlangBio. Additionally, the PRC Subsidiary agrees to grant SenlangBio its trademarks, software copyrights, management systems, management methods and other intellectual property in relation to its services on a chargeable and revocable basis, but such grant shall not result in the transfer of any intellectual property or create any restriction on the PRC Subsidiary’s full ownership.
For services rendered to SenlangBio under this agreement, the PRC Subsidiary is entitled to collect a service fee calculated based on the complexity of services rendered, time required by the PRC Subsidiary, and the exact contents and commercial value of services rendered. During the term of this agreement, the PRC Subsidiary shall enjoy all the economic benefits derived from SenlangBio’s operations, and in the event of serious difficulties in SenlangBio’s operations, the PRC Subsidiary may provide SenlangBio with financial support, and the PRC Subsidiary has the right to request SenlangBio to cease operation. The Exclusive Technical Consultation and Service Agreement shall remain in effect for ten (10) years and shall be automatically renewed unless it is terminated earlier by the PRC Subsidiary.
Based on the foregoing VIE Agreements, the PRC Subsidiary has effective control of SenlangBio which enables the PRC Subsidiary to receive all of the expected residual returns and absorb the expected losses of the VIE and its subsidiary. Sen Lang’s management therefore concluded that Sen Lang, through the above contractual arrangements, has the power to direct the activities that most significantly impact the VIE’s economic performance, bears the risks of and enjoys the rewards normally associated with ownership of the VIE, and therefore Sen Lang is the ultimate primary beneficiary of the VIE. Consequently, Sen Lang consolidates the accounts of SenlangBio and its subsidiary for the periods presented in the financial statements of Sen Lang included in this proxy statement, in accordance with Accounting Standards Codification (“ASC”) 810-10, Consolidation.
Interests of Avalon’s Directors and Officers in the Acquisition
In considering the recommendation of Avalon’s board of directors with respect to issuing shares of Avalon Common Stock as contemplated by the Purchase Agreement and the other matters to be acted upon by Avalon stockholders at the Avalon annual meeting, Avalon stockholders should be aware that certain members of the board of directors and executive officers of Avalon have interests in the Acquisition that may be different from, or in addition to, the interests of Avalon stockholders. These interests relate to or arise from the matters described below. The board of directors of Avalon was aware of these potential conflicts of interest and considered them, among other matters, in reaching its decision to approve the Purchase Agreement and the transactions contemplated thereby, and to recommend that Avalon stockholders approve the Avalon Proposals to be presented to Avalon stockholders for consideration at the Avalon annual meeting as contemplated by this proxy statement.
Continued Service
As described elsewhere in this proxy statement, including in the section entitled “Management After the Acquisition,” all of the current executive officers of Avalon will continue in their current positions after the Acquisition, and all of the directors except for Meng Li will continue on the Avalon board after the Acquisition.
Stock Ownership
As of June 30, 2021, Avalon’s directors and executive officers beneficially owned approximately 66.3% of the shares of Avalon Common Stock (calculated in accordance with SEC rules that define beneficial ownership) and owned 54,145,161 shares, 63.6% of the issued and outstanding Avalon Common Stock on such date. Although under no contractual or other obligation to do so, all of such directors and executive officers are currently expected to vote in favor of all of the Avalon Proposals, including the Nasdaq Proposal.
Affiliation with Beijing Lu Daopei Hospital
On April 10, 2020, in the ordinary course of business, SenlangBio entered into a scientific research project cooperation agreement with Beijing Lu Daopei Hospital Co., Ltd., under which Beijing Lu Daopei Hospital Co., Ltd. conducts scientific research for the interest of SenlangBio on the cytoplasmic CD79a antibody gated multicolor flow cytometry monitoring CD19-CAR-T bridging allogeneic transplantation for the treatment of refractory and relapsed acute B lymphocytic leukemia. SenlangBio provides research funds in the amount of RMB 2 million to Beijing Lu Daopei Hospital Co., Ltd. Beijing Lu Daopei Hospital Co., Ltd. is a wholly-owned subsidiary of an entity whose chairman is Wenzhao Lu, the Chairman and largest shareholder of Avalon.
49
Indemnification and Insurance
As described in this proxy statement, including in the section entitled “Management After the Acquisition—Limitation on Liability and Indemnification of Directors and Officers,” certain of Avalon’s directors and officers will be entitled to certain ongoing rights of indemnification and coverage under directors’ and officers’ liability insurance policies.
The Avalon board of directors was aware of these interests and considered them, among other matters, in its decision to approve the Acquisition.
Regulatory Approvals Required for the Acquisition
In the United States, Avalon must comply with applicable federal and state securities laws and the rules and regulations of Nasdaq in connection with the issuance of shares of Avalon Common Stock in connection with the Acquisition and the issuance of the Exchange Shares in the Equity Financing and the filing of this proxy statement with the SEC.
Accounting Treatment of the Acquisition
The Acquisition is expected to be accounted for as a business acquisition, with Avalon identified as the accounting acquirer. Avalon is considered the accounting acquirer since immediately following the closing: (i) Avalon stockholders will own a majority of the voting rights of the post-Acquisition company; (ii) Avalon will have designate a majority (eight of nine) of the initial members of the board of directors of the post-Acquisition company; (iii) Avalon’s senior management will hold the majority of the key positions in senior management of the post-Acquisition company; and (iv) Avalon will continue to maintain its corporate headquarters in Freehold, New Jersey, United States. SenlangBio will continue to maintain operations in the Shijiazhuang High-tech Development Zone, Hebei Province, China.
The acquisition consideration is 81,000,000 shares of Avalon Common Stock. The purchase price will be allocated to the acquired assets and assumed liabilities based on their fair values at the closing date, and any excess is initially allocated to identifiable intangible assets mainly consisting of cell and gene engineering technologies with the ability to generate innovative and transformative cellular immunotherapies for solid and hematologic cancers, which will be amortized over 10 years. The initial allocation is subject to change upon the final valuation which is to be done at the time of closing. Such change could have a material impact on Avalon’s financial statements.
Appraisal Rights and Dissenters’ Rights
No appraisal or dissenters’ rights are available to holders of shares of Avalon Common Stock in connection with the Acquisition.
50
The following is a summary of the material terms of the Purchase Agreement. A copy of the Purchase Agreement is attached as Annex A to this proxy statement and is incorporated by reference into this proxy statement. The Purchase Agreement has been attached to this proxy statement to provide you with information regarding its terms. It is not intended to provide any other factual information about Avalon, Sen Lang or the other parties thereto. The following description does not purport to be complete and is qualified in its entirety by reference to the Purchase Agreement. You should refer to the full text of the Purchase Agreement for details of the Acquisition and the terms and conditions of the Purchase Agreement.
The Purchase Agreement contains representations and warranties that Avalon, on the one hand, and the Sen Lang Owners, on the other hand, have made to one another as of specific dates. These representations and warranties have been made for the benefit of the other parties to the Purchase Agreement and may be intended not as statements of fact but rather as a way of allocating the risk to one of the parties if those statements prove to be incorrect. In addition, the assertions embodied in the representations and warranties are qualified by information in confidential disclosure schedules exchanged by the parties in connection with signing the Purchase Agreement. While Avalon does not believe that these disclosure schedules contain information required to be publicly disclosed under the applicable securities laws, other than information that has already been so disclosed, the disclosure schedules do contain information that modifies, qualifies and creates exceptions to the representations and warranties set forth in the attached Purchase Agreement. Accordingly, you should not rely on the representations and warranties as current characterizations of factual information about Avalon or the Acquired Companies, because they were made as of specific dates, may be intended merely as a risk allocation mechanism among the parties to the Purchase Agreement and are modified by the disclosure schedules.
General
On June 13, 2021, Avalon GloboCare Corp., a Delaware corporation (the “Company” or “Avalon”), entered into a Share Purchase Agreement (the “Purchase Agreement”), by and among the Company, Lonlon Biotech Ltd., a company incorporated in the British Virgin Islands (“BVI”) (“Sen Lang”), the holders of the share capital of Sen Lang (the “Sen Lang Shareholders”), the ultimate beneficial owners of the Sen Lang Shareholders (the “Sen Lang Beneficial Shareholders” and, together with the Sen Lang Shareholders, the “Sen Lang Owners”) and a representative of the Sen Lang Owners (the “Sen Lang Representative”). Pursuant to the Purchase Agreement, Avalon agreed to purchase (the “Acquisition”) all of the issued and outstanding share capital of Sen Lang (the “Sen Lang Shares”).
Acquisition Consideration
The purchase price being paid by Avalon to the Sen Lang Shareholders under the Purchase Agreement for the Sen Lang Shares is an aggregate of 81 million shares (the “Acquisition Shares”) of the common stock, par value US$0.0001 per share, of Avalon (the “Avalon Common Stock”). Ten percent (10%), or 8.1 million, of such shares will be held in escrow for 12 months following the closing to satisfy any indemnification obligations of the Sen Lang Shareholders under the Share Purchase Agreement. The Acquisition Shares will not be registered under the Securities Act of 1933, as amended (the “Securities Act”) and, therefore, will be restricted securities under Rule 144 under the Securities Act for six months or longer after the closing of the Acquisition, subject to “affiliate” status with the Company under the Securities Act.
Directors and Executive Officers of the Post-Acquisition company Following the Acquisition
Upon the closing of the Acquisition, it is currently expected that Dr. Jianqiang Li, scientific founder and CSO of SenlangBio, will join the board of Avalon, and Dr. Li will also be appointed as Chief Technology Officer of Avalon. In addition, upon the closing the Acquisition, Meng Li, Avalon’s Chief Operating Officer and a director, will resign from her position on the board of Avalon. The Avalon board and management will otherwise remain the same aside from Dr. Li, with Wenzhao Lu (Chairman), David Jin, MD, PhD, Steven A. Sanders, Yancen Lu, Wilbert J. Tauzin II, Willliam B. Stilley, III, Tevi Troy and Yue “Charles” Li continuing to serve on the board and Dr. Jin continuing to serve as President and Chief Executive Officer, Ms. Li as Chief Operating Officer and Luisa Ingargiola as Chief Financial Officer.
51
Conditions to the Closing of the Acquisition
Each party’s obligation to complete the Acquisition is subject to the satisfaction or waiver by each of the parties, at or prior to the closing of the Acquisition (the “Closing”), of various conditions, which include, in addition to other customary closing conditions, the following:
| ● | there shall not have been issued any statute, rule, regulation, judgment, decree, injunction or other order prohibiting the Closing by any governmental entity of competent jurisdiction or which would have a material adverse effect on Avalon after the Closing or after giving effect to the Acquisition; |
| ● | the Nasdaq Proposal shall have been approved by the Avalon stockholders; and |
| ● | the Acquisition shares shall have been approved for listing (subject to official notice of issuance) on Nasdaq. |
In addition, the obligation of Avalon to complete the Acquisition is further subject to the satisfaction or waiver of the following conditions, among others set forth in the Purchase Agreement:
| ● | certain fundamental representations and warranties of the Acquired Companies shall have been true and correct in all respects on the date of the Purchase Agreement and shall be true and correct on the date of the Closing (the “Closing Date”) of the Acquisition with the same force and effect as if made on and as of the date on which the Acquisition is to be completed or, if such representations and warranties address matters as of a particular date, then such fundamental representations and warranties shall be true and correct as of that particular date; |
| ● | all other representations and warranties of the Acquired Companies in the Purchase Agreement shall have been true and correct as of the date of the Purchase Agreement and shall be true and correct on the Closing Date of the Acquisition with the same force and effect as if made on the date on which the Acquisition is to be completed or, if such representations and warranties address matters as of a particular date, then such representations and warranties shall be true and correct as of that particular date, except where the failure of these representations and warranties to be true and correct, individually or in the aggregate, would not reasonably be expected to have a material adverse effect on the Acquired Companies, taken as a whole (a “Sen Lang Material Adverse Effect”); |
| ● | the Acquired Companies shall have performed or complied with in all material respects all of its covenants and agreements in the Purchase Agreement required to be performed or complied with by it on or before the Closing of the Acquisition; |
| ● | since the date of the Purchase Agreement, there shall not have occurred any act, event or omission having or reasonably likely to have a Sen Lang Material Adverse Effect; |
| ● | The Acquired Companies shall have obtained all necessary consents and approvals for the performance of the Purchase Agreement; |
| ● | The Equity Financing shall have been consummated no later than concurrently with the Closing; |
| ● | the Acquired Companies shall have delivered certain certificates and other documents required under the Purchase Agreement for the Closing; and |
| ● | all of the necessary VIE Agreements shall have been executed and delivered to the satisfaction of Avalon. |
In addition, the obligation of the Acquired Companies and the Sen Lang Owners to complete the Acquisition is further subject to the satisfaction or waiver of the following conditions, among others set forth in the Purchase Agreement:
| ● | certain fundamental representations and warranties of Avalon shall have been true and correct in all respects on the date of the Purchase Agreement and shall be true and correct on the Closing Date of the Acquisition with the same force and effect as if made on and as of the date on which the Acquisition is to be completed or, if such representations and warranties address matters as of a particular date, then such fundamental representations and warranties shall be true and correct as of that particular date; |
52
| ● | all other representations and warranties of Avalon in the Purchase Agreement shall have been true and correct as of the date of the Purchase Agreement and shall be true and correct on the Closing Date of the Acquisition with the same force and effect as if made on the date on which the Acquisition is to be completed or, if such representations and warranties address matters as of a particular date, then such representations and warranties shall be true and correct as of that particular date, except where the failure of these representations and warranties to be true and correct, individually or in the aggregate, would not reasonably be expected to have a material adverse effect on Avalon (an “Avalon Material Adverse Effect”); |
| ● | Avalon shall have performed or complied with in all material respects all of its covenants and agreements in the Purchase Agreement required to be performed or complied with by it on or before the Closing of the Acquisition; |
| ● | Avalon shall have obtained all necessary consents and approvals for the performance of the Purchase Agreement; |
| ● | Avalon shall have delivered certain certificates and other documents required under the Purchase Agreement for the Closing of the Acquisition; and |
| ● | since the date of the Purchase Agreement, there shall not have occurred any act, event or omission having or reasonably likely to have an Avalon Material Adverse Effect. |
Non-Solicitation
In the Purchase Agreement the Acquired Companies agreed not to (i) solicit, initiate, or encourage the submission of any proposal or offer from any person relating to the acquisition of the equity or assets of the Acquired Companies or (ii) enter into or renew any distribution agreement related to the business of the Acquired Companies, in each case without the prior written consent of Avalon.
Stockholder Approvals
Avalon is obligated under the Purchase Agreement to, as promptly as practicable hold an annual meeting of the holders of Avalon Common Stock to consider and vote to approve the issuance of the Acquisition Shares.
Sen Lang is obligated under the Purchase Agreement to obtain any and all necessary approvals of the Sen Lang Owners for the consummation of the Acquisition.
Covenants; Conduct of Business Pending the Acquisition
The Acquired Companies and the Sen Lang Owners (collectively, the Sen Lang Parties”) have agreed not to:
| ● | take any action or omit to take any action which would result in any Acquired Company (a) incurring any trade accounts payable outside of the ordinary course of business or making any commitment to purchase quantities of any item of inventory in excess of quantities normally purchased in the ordinary course of business; (b) increasing any of its indebtedness for borrowed money except in the ordinary course of business; (c) guaranteeing the obligations of any entity other than an Acquired Company, (d) making any purchases of pharmaceuticals other than from the manufacturers thereof or wholesalers or other distributors authorized by such manufacturers to distribute their products; (e) merging or consolidating with, purchasing substantially all of the assets of, or otherwise acquiring any business or any proprietorship, firm, association, limited liability company, corporation or other business organization; (f) increasing or decreasing the rate or type of compensation payable to any officer, director, employee or consultant of any Acquired Company (other than regularly scheduled increases in base salary and annual bonuses consistent with prior practice); (g) entering into or amending any collective bargaining agreement, or creating or modifying any pension or profit-sharing plan, bonus, deferred compensation, death benefit, or retirement plan, or any other employee benefit plan, or increasing the level of benefits under any such plan, or extending the exercisability of any outstanding stock option or increasing or decreasing any severance or termination pay benefit or any other fringe benefit; (h) making any representation to anyone indicating any intention of Avalon or its Subsidiaries to retain, institute, or provide any employee benefit plans; (i) declaring or paying any dividend or making any distribution with respect to, or purchasing or redeeming, shares of the capital stock of any Acquired Company; (j) selling, licensing or disposing of any assets otherwise than in the ordinary course of business of the Acquired Companies; (k) making any capital expenditures other than in the ordinary course of business consistent with past practices and in no event in excess of $50,000 in the aggregate; (l) issuing any shares of the capital stock of any kind of any Acquired Company, transferring from the treasury of any Acquired Company any shares of the capital stock of any Acquired Company or issuing or granting any subscriptions, options, rights, warrants, convertible securities or other agreements or commitments to issue, or contracts or any other agreements obligating any Acquired Company to issue, or to transfer from treasury, any shares of capital stock of any class or kind, or securities convertible into any such shares; (m) modifying, amending or terminating any material contract other than in the ordinary course of business that is consistent with past practices; or (n) entering into any other transaction outside of the ordinary course of business; |
53
| ● | change any method or principle of accounting in a manner that is inconsistent with past practice, except to the extent required by generally accepted accounting principles as advised by Sen Lang’s regular independent accountants; |
| ● | make, change or revoke any material tax election, fail to pay any income or other material tax as such tax becomes due and payable, file any amendment making any material change to any tax return, settle or compromise any income or other material tax liability, enter into any tax allocation, sharing, indemnification or other similar agreement or arrangement, request or consent to any extension or waiver of any limitation period with respect to any claim or assessment for any income or other material taxes (other than in connection with any extension of time to file any tax return), or adopt or change any accounting method in respect of taxes; |
| ● | take any action that would likely result in the representations and warranties set forth the Purchase Agreement as made by the Sen Lang Parties (other than representations made as of a particular date) becoming false or inaccurate in any material respect (or, as to representations and warranties, which, by their terms, are qualified as to materiality, becoming false or inaccurate in any respect); |
| ● | incur or create any encumbrances, liens, pledges or security interests on assets other than permitted encumbrances; |
| ● | except as contemplated in the Purchase Agreement, take any action or omit to take any action which would materially interfere with Avalon’s rights to compel performance of each of the obligations of Sen Lang under the Purchase Agreement; |
| ● | take or omit to be taken any action, or permit any of its affiliates to take or to omit to take any action, which would reasonably be expected to result in a Sen Lang Material Adverse Effect; or |
| ● | agree or commit to take any action set forth above. |
Avalon has agreed not to:
| ● | change any method or principle of accounting in a manner that is inconsistent with past practice, except to the extent required by generally accepted accounting principles as advised by Avalon’s regular independent accountants; |
| ● | take any action that would likely result in the representations and warranties set forth in the Purchase Agreement as made by Avalon (other than representations made as of a particular date) becoming false or inaccurate in any material respect (or, as to representations and warranties, which, by their terms, are qualified as to materiality, becoming false or inaccurate in any respect); |
| ● | except as contemplated in the Purchase Agreement, take any action or omit to take any action which would materially interfere with Sen Lang’s rights to compel performance of each of the obligations of Avalon under the Purchase Agreement; |
| ● | take or omit to be taken any action, or permit any of its affiliates to take or to omit to take any action, which would reasonably be expected to result in an Avalon Material Adverse Effect; |
| ● | amend Avalon’s certificate of incorporation or by-laws, each as amended, in any material manner that does not generally apply to all of Avalon’s stockholders; or |
| ● | agree or commit to take any action set forth above. |
54
Other Agreements
Each of Avalon and the Sen Lang Parties has agreed to use its commercially reasonable efforts to, among other things:
| ● | cause this proxy statement to comply with the rules and regulations promulgated by the SEC, to respond promptly to any comments of the SEC or its staff and to clear this proxy statement with the SEC as promptly as practicable after it is filed with the SEC; |
| ● | satisfy the conditions precedent to the consummation of the transactions contemplated by the Purchase Agreement; and |
| ● | provide the other party with reasonable access to such party’s personnel and assets and to all existing books, records, tax returns, work papers and other documents and information relating to such party and its subsidiaries. |
Indemnification
The Sen Lang Owners agreed to indemnify Avalon and its affiliates from any damages arising from any breach, inaccuracy or nonfulfillment or the alleged breach, inaccuracy or nonfulfillment of any of the representations, warranties and covenants of Sen Lang and the Sen Lang Owners contained in the Purchase Agreement or from the VIE Agreements not being found valid or enforceable. The indemnification obligations are capped at the value of approximately 8,100,000 of the Acquisition Shares that will be held in escrow, except for claims related to fraud and certain fundamental representations, in which case the obligations are capped at the amount of the Acquisition Shares actually paid by Avalon to the indemnifying party.
Termination of the Purchase Agreement
The Purchase Agreement may be terminated and/or abandoned at any time prior to the closing, whether before or after approval of the proposals being presented to Avalon’s stockholders, under certain circumstances, including by:
| ● | the Sen Lang Representative if the Board of Directors of Avalon shall withdraw, modify or change the Company Board Recommendation in a manner adverse to the Sen Lang Owners; |
| ● | either Avalon or the Sen Lang Representative if at the Avalon Annual Meeting (as defined above) the requisite vote of Avalon’s stockholders to authorize the issuance of the Acquisition Shares in the Acquisition in accordance with the rules of Nasdaq shall not have been obtained; |
| ● | Avalon if any authorization, consent, waiver or approval required for the consummation of the Acquisition shall require the divestiture or cessation of any of the present business or operations conducted by Avalon or its subsidiaries or any Acquired Company or shall impose any other material condition or requirement, which divestiture, cessation, condition or requirement, in the reasonable judgment of Avalon’s Board of Directors, would be reasonably likely to have an Avalon Material Adverse Effect after the Closing giving effect to consummation of the Acquisition; and |
| ● | either Avalon or the Sen Lang Representative if the closing has not occurred by December 31, 2021. |
Amendment
No amendment of any provision of the Purchase Agreement shall be valid unless the same shall be in writing and signed by Avalon and the Sen Lang Representative.
55
In connection with the Acquisition, on June 13, 2021, an institutional investor (the “Investor”) entered into an agreement, as amended on June 24, 2021, with SenlangBio related to the purchase of registered capital of SenlangBio (the “OpCo Capital Increase Agreement”) pursuant to which the Investor will acquire an aggregate of up to 13.5% of the equity ownership of SenlangBio for an aggregate purchase price (the “Subscription Amount) of approximately US$30,000,000 (represented by an actual investment of RMB200,000,000) (the “Equity Financing”), which funds will be invested in SenlangBio in three equal installments of approximately US$10,000,000 (represented by actual installments of RMB67,000,000, RMB67,000,000 and RMB66,000,000), at a fixed price, the first to be upon the closing of the Acquisition, the second to be within three months after the closing and the third to be within six months after the closing. In addition, pursuant to a Securities Exchange Agreement, as amended on June 24, 2021 (the “Exchange Agreement”), by and among the Company, Sen Lang, SenlangBio and the Investor, dated June 13, 2021, the Investor shall have the right, exercisable between the six-month and five year-anniversaries of the respective initial closing and installment closings, to elect to exchange, from time to time, all or part of its then-owned equity ownership of SenlangBio for shares (the “Exchange Shares”) of Avalon Common Stock at a fixed exchange price of US$1.21 per share of Avalon Common Stock, which was the market price of the Avalon Common Stock as of the date of the Exchange Agreement under Nasdaq rules. In addition, the Exchange Agreement provides that the Investor may only exchange up to 10% of its total investment amount in any 30 day period.
The Investor is entitled to receive a number of Exchange Shares equal to the portion of the Subscription Amount (which is measured in RMB) being exchanged (i) converted into US dollars at the RMB exchange rate on the date of the exchange notice and (ii) divided by the exchange price of $1.21. Such exchange price will be adjusted to reflect any stock split, reclassification, combination or other similar change of Avalon Common Stock.
In addition, between the six-month and five year-anniversaries of the respective initial closing and installment closings, the Investor shall have the right to elect to exchange, from time to time, all or part of its then-owned equity ownership of SenlangBio for shares of Sen Lang by taking the portion of the Subscription Amount (which is measured in RMB) being exchanged and (i) converting it into US dollars at the RMB exchange rate on the date of the exchange notice and (ii) dividing it by the fixed exchange price of $20,014.4918 per share of Sen Lang, subject to adjustment for any stock split, reclassification, combination or other similar change.
The conditions to the initial closing include the material accuracy of representations and warranties contained in the Exchange Agreement and the OpCo Capital Increase Agreement, that performance of all applicable covenants and agreements by the parties, the consummation of the Acquisition, the execution by the Investor of the VIE Agreements, the approval of the issuance of the Exchange Shares by the Avalon stockholders pursuant to the Nasdaq Proposal and the approval of the listing of the Exchange Shares on Nasdaq, subject to official notice of issuance.
Avalon has no plans, and is under no obligation, to register the Exchange Shares under the Securities Act, and, therefore, such shares will be restricted securities under Rule 144 under the Securities Act.
56
PROPOSAL NO. 1—THE DIRECTOR ELECTION PROPOSAL
At the annual meeting, the Avalon Board of Directors proposes that the nominees listed below be elected to hold office until the next annual meeting of stockholders and until their successors are duly elected and qualified. All of the nominees are currently serving as directors. All nominees have consented to being named in this proxy statement and to serve if elected. As described above, it is currently anticipated that upon the closing of the Acquisition, Meng Li, Avalon’s Chief Operating Officer and a director, will resign from her position on the board of Avalon.
Assuming a quorum is present, the nine nominees receiving the highest number of affirmative votes of shares entitled to be voted for such persons will be elected as directors of Avalon to hold office until the next annual meeting of stockholders and until their successors are duly elected and qualified. Unless marked otherwise, proxies received will be voted “FOR” the election of the nominees named below. In the event that additional persons are nominated for election as directors, the proxy holders intend to vote all proxies received by them in such a manner as will ensure the election of the nominees listed below, and, in such event, the specific nominees to be voted for will be determined by the proxy holders.
Information With Respect to Director Nominees
Listed below are the nominees for election to the Avalon Board with information showing the principal occupation or employment of the nominees for director, the principal business of the corporation or other organization in which such occupation or employment is carried on, and such nominees’ business experience during the past five years. Such information has been furnished to Avalon by the director nominees.
| Name | Age | Position | ||
| Wenzhao “Daniel” Lu | 63 | Chairman of the Board of Directors | ||
| David Jin, MD, PhD | 53 | Chief Executive Officer, President and Director | ||
| Meng Li | 43 | Chief Operating Officer, Secretary and Director | ||
| Steven A. Sanders | 75 | Director | ||
| Yancen Lu | 46 | Director | ||
| Wilbert J. Tauzin II | 76 | Director | ||
| William B. Stilley, III | 53 | Director | ||
| Tevi Troy | 53 | Director | ||
| Yue “Charles” Li | 47 | Director |
The principal occupation and business experience during at least the past five years for the directors is as follows:
Wenzhao “Daniel” Lu, Chairman of the Board of Directors
Mr. Wenzhao Lu is Avalon’s Chairman of the Board. He is a seasoned healthcare entrepreneur with extensive operational knowledge and experience in China. He has been serving as Chairman of the Board for the Daopei Medical Group, or DPMG, since 2010. Under his leadership, DPMG has recently expanded its clinical network involving a state-of-the-art stem cell bank at Wuhan Biolake, three top-ranked private hospitals (located in Beijing, Shanghai, and Hebei), specialty hematology laboratories, as well as a hematology research institute, with more than 100 partnering and collaborating hospitals in China. DPMG was founded by Professor Daopei Lu, a renowned hematologist pioneering in hematopoietic stem cell transplant and member of the Academy of Engineering in China. Mr. Wenzhao Lu received a Bachelor of Arts from Temple University Tyler School of Arts in 1988 and subsequently worked as senior Art Director at Ogilvy & Mather Advertising Company. Prior to joining DPMG, Mr. Lu served as Chief Operating Officer for BioTime Asia Limited, which is a subsidiary of BioTime, Inc. (NYSE American: BTX) in 2009. Mr. Lu is qualified to serve as a director because of his extensive operational knowledge of, and executive level management experience in, the healthcare industry.
57
David Jin, Chief Executive Officer, President and Director
Dr. David Jin, MD, PhD, is Avalon’s Chief Executive Officer, President and a member of the Board of Directors. From 2009 to 2017, Dr. Jin has served as the Chief Medical Officer of BioTime, Inc. (NYSE American: BTX), a clinical stage regenerative medicine company with a focus on pluripotent stem cell technology. Dr. Jin also acts as a senior translational clinician-scientist at the Howard Hughes Medical Institute and the Ansary Stem Cell Center at Weill Cornell Medical College of Cornell University. Prior to his current endeavors, Dr. Jin was Chief Consultant/Advisor for various biotech/pharmaceutical companies regarding hematology, oncology, immunotherapy and stem cell-based technology development. Dr. Jin has been Principle Investigator in more than 15 pre-clinical and clinical trials, as well as author/co-author of over 80 peer-reviewed scientific abstracts, articles, reviews, and book chapters. Dr. Jin studied medicine at SUNY Downstate College of Medicine in Brooklyn, New York. He received his clinical training and subsequent faculty tenure at the New York-Presbyterian Hospital (the teaching hospital for both Cornell and Columbia Universities) in the areas of internal medicine, hematology, and clinical oncology. Dr. Jin was honored as Top Chief Medical Officer by ExecRank in 2012, as well as recognized by Leading Physicians of the World in 2015. Dr. Jin is qualified to serve as a director because of his role with Avalon and his extensive operational knowledge of, and executive level management experience in, the healthcare industry.
Meng Li, Chief Operating Officer, Secretary and Director
Ms. Meng Li is Avalon’s Chief Operating Officer and Secretary and a member of the Board of Directors. Previously, Ms. Li served on the Board of Directors from October 2017 through July 2018 and was re-appointed in February 2019. Ms. Li has over 15 years of executive experience in international marketing, branding, communications, and media investment consultancy. Ms. Li served as Managing Director at Maxus/GroupM (a WPP Group company) where she was responsible for business P&L and corporate management from 2006 to 2015. Prior to joining Maxus/Group M, Ms. Li worked for Zenith Media (a Publicis Group company) from 2000 to 2006 as Senior Manager. Ms. Li received a Bachelor of Arts in International Economic Law from Dalian Maritime University in China. Ms. Li is qualified to serve as a director because of her role with Avalon and her executive level management experience.
Upon the closing of the Acquisition, it is expected that Ms. Li will resign from her position on the board of Avalon, and Dr. Jianqiang Li will be appointed to the board.
Steven A. Sanders, Director
Steven A. Sanders is a member of the Board of Directors. Since January 2017, Mr. Sanders has been Of Counsel to the law firm of Ortoli Rosenstadt LLP. From July 2007 until January 2017, Mr. Sanders was a Senior Partner of Ortoli Rosenstadt LLP. From January 1, 2004 until June 30, 2007, he was Of Counsel to the law firm of Rubin, Bailin, Ortoli, LLP. From January 1, 2001 to December 31, 2003, he was Counsel to the law firm of Spitzer & Feldman PC. Mr. Sanders also serves as a Director of Helijet International, Inc. and Electrameccanica Vehicles Corp. (NASDAQ:SOLO). Additionally, he has been a director at the American Academy of Dramatic Arts since October 2013 and has been a director of the Bay Street Theater since February 2015. Mr. Sanders received his JD from Cornell University and his BBA from The City College of New York. Mr. Sanders is qualified to serve as a director because of his corporate, securities and international law experience, including working with companies in the life sciences industry.
Yancen Lu, Director
Yancen Lu is a member of the Board of Directors. Mr. Lu has more than 20 years of experience in investment banking and equity investment management. He is the Founder and CEO of PagodaTree Partners, a healthcare PE fund. Before this, Mr. Lu was the Managing Director of FountainVest Partners. In addition to his professionalism in securities, investment and capital management, Mr. Lu has a special focus and comprehensive understanding of the global medical and healthcare industry. He served as Director of leading healthcare corporations including Sino Hospital Investment Corporation (Hong Kong), Chang’an Hospital (the largest private hospital in Northwest China), and DIH Medical Technologies. Mr. Lu received Bachelor’s and Master’s degrees in Engineering Economics from Tianjin University. Mr. Lu is qualified to serve as a director because of his extensive operational knowledge of, and executive level management experience in, the healthcare industry.
58
Wilbert J. Tauzin II, Director
Wilbert J. Tauzin II is a member of the Board of Directors. From December 2010 until March 1, 2014, Congressman Tauzin served as Special Legislative Counsel to Alston & Bird LLP. From December 2004 to June 2010, Congressman Tauzin was President and Chief Executive Officer of the Pharmaceutical Research and Manufacturers of America, a trade group that serves as one of the pharmaceutical industry’s top lobbying groups. He served 12.5 terms in the U.S. House of Representatives, representing Louisiana’s 3rd Congressional District. From January 2001 through February 2004, Congressman Tauzin served as Chairman of the House Committee on Energy and Commerce. He also served as a senior member of the House Resources Committee and Deputy Majority Whip. Prior to serving as a member of Congress, Congressman Tauzin was a member of the Louisiana State Legislature, where he served as Chairman of the House Natural Resources Committee and Chief Administration Floor Leader. He currently serves as lead independent director of LHC Group, a publicly traded provider of quality home health care. Congressman Tauzin received a Bachelor of Arts Degree from Nicholls State University and a Juris Doctor degree from Louisiana State University. Congressman Tauzin is qualified to serve as a director because of his extensive knowledge of the pharmaceutical industry and his experience as a director of several publicly-traded and privately-held companies.
William B. Stilley, III, Director
William B. Stilley is a member of the Board of Directors. Mr. Stilley has been the chief executive officer and member of the board of directors of Adial Pharmaceuticals, Inc. since December 2010. From August 2008 until December 2010, he was the vice president, business development and strategic projects at Clinical Data, Inc. (NASDQ: CLDA). From February 2002, Mr. Stilley was the COO and CFO of Adenosine Therapeutics, LLC until certain assets of Adenosine Therapeutics were acquired by Clinical Data, Inc. in August 2008. Mr. Stilley has advised both public and private companies on financing and M&A transactions, has been the interim CFO of a public company, the interim Chief Business Officer and then Advisor for Diffusion Pharmaceuticals from September 2015 through March 2018, and the COO and CFO of a number of private companies. Before entering the business community, Mr. Stilley served as Captain in the U.S. Marine Corps. Mr. Stilley has an MBA with honors from the Darden School of Business and a B.S. in Commerce/Marketing from the McIntire School of Commerce at the University of Virginia. He currently serves on the Advisory Board of Virginia BIO, the statewide biotechnology organization. Mr. Stilley is qualified to serve as a director because of his extensive knowledge of the biotechnology industry, significant executive leadership and operational experience, and knowledge of, and experience in, financing and M&A transactions.
Tevi Troy, Director
Tevi Troy is a member of the Board of Directors and a former Deputy Secretary of the U.S. Department of Health and Human Services. Dr. Troy has previously been the founder and CEO of the American Health Policy Institute and a Senior Fellow at Hudson Institute, where he remains an Adjunct Fellow. On August 3, 2007, Dr. Troy was unanimously confirmed by the U.S. Senate as the Deputy Secretary of HHS. As Deputy Secretary, Dr. Troy was the chief operating officer of the largest civilian department in the federal government, with a budget of $716 billion and over 67,000 employees. Dr. Troy has extensive White House experience, having served in several high-level positions over a five-year period, culminating in his service as Deputy Assistant and then Acting Assistant to the President for Domestic Policy. Dr. Troy has held high-level positions on Capitol Hill as well. From 1998 to 2000, Dr. Troy served as the Policy Director for Senator John Ashcroft. From 1996 to 1998, Dr. Troy was Senior Domestic Policy Adviser and later Domestic Policy Director for the House Policy Committee, chaired by Christopher Cox. In addition to his senior level government work and health care expertise, Dr. Troy is also a best-selling presidential historian and the author of five books, including, most recently, "Fight House: Rivalries in the White House from Truman to Trump," which the Wall Street Journal listed as one of the top political books of 2020. Dr. Troy’s many other affiliations include: contributing editor for Washingtonian magazine; member of the publication committee of National Affairs; member of the Board of Fellows of the Jewish Policy Center; a Senior Fellow at the Potomac Institute; and a member of the Bipartisan Commission on Biodefense. Dr. Troy has a B.S. in Industrial and Labor Relations from Cornell University and an M.A and Ph.D. in American Civilization from the University of Texas at Austin. Dr. Troy is qualified to serve as a director because of his extensive knowledge of the healthcare industry and his significant leadership experience.
59
Yue “Charles” Li
Yue “Charles” Li is a member of the Board of Directors. Mr. Li has about 20 years of experience in M&A and capital markets in China and the U.S. Mr. Li currently is a Managing Director at PagodaTree Partners, a private equity company with a focus on healthcare in Beijing. Prior to PagodaTree, he was a senior executive at a major conglomerate in China where he successfully closed $2 billion M&A transactions in healthcare and insurance areas. Previously, Mr. Li spent 8 years in Deloitte, as a director of financial advisory services in Beijing and capital markets in New York. His key clients included Merrill Lynch, Blackrock, KKR etc. In his early career, Mr. Li served for top tier financial institutions such as Credit Suisse and Fannie Mae, responsible for asset allocation strategy and risk management for multibillion USD portfolios. Mr. Li received Master’s degree from the Olin School of Business at Washington University in 2000 and a Bachelor of Engineering from Tianjin University in 1996. He is a CFA charter holder. Mr. Li is qualified to serve as a director because of his extensive investment and executive level management experience.
Required Vote
The election of the directors of the Company requires the affirmative vote of a plurality of the shares of the Company’s common stock present or represented by proxy at the annual meeting, which will be the nominees receiving the largest number of votes, which may or may not constitute a majority.
AVALON’S BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT ITS STOCKHOLDERS VOTE “FOR” THE ELECTION OF THE DIRECTORS SET FORTH IN THE DIRECTOR ELECTION PROPOSAL.
60
PROPOSAL NO. 2—THE AUDITOR PROPOSAL
The audit committee of Avalon’s board of directors has appointed Marcum LLP (“Marcum”) as the Company’s independent registered public accounting firm to audit its consolidated financial statements for the fiscal years ending December 31, 2021. At the annual meeting, stockholders will be asked to ratify the appointment of Marcum as the Company’s independent registered public accounting firm for the year ending December 31, 2021. Stockholder ratification of the appointment of the independent registered public accounting firm is not required by the Company’s bylaws or other applicable legal requirements. However, Avalon’s board of directors submits the appointment of Marcum to stockholders for ratification as a matter of good corporate governance. If this appointment is not ratified by the affirmative vote of the holders of a majority of votes cast by the stockholders present in person or represented by proxy and entitled to vote thereon at the annual meeting, the appointment will be reconsidered by our audit committee. Even if the appointment is ratified, Avalon’s audit committee, in its sole discretion, may appoint another independent registered public accounting firm at any time during the fiscal year ending December 31, 2021 if the audit committee believes that such a change would be in the best interests of the Company and its stockholders. A representative of Marcum is expected to be present at the Annual Meeting, will have an opportunity to make a statement if he or she wishes to do so, and is expected to be available to respond to appropriate questions from stockholders.
Fees Paid to Independent Registered Public Accounting Firm
Marcum served as Avalon’s independent auditors for the years ended December 31, 2020 and 2019. Aggregate fees billed to the Company for professional services rendered by Marcum during the last two fiscal years were as follows:
| December 31, 2020 | December 31, 2019 | |||||||
| Audit Fees | $ | 252,144 | $ | 186,669 | ||||
| Audit Related Fees | - | - | ||||||
| Tax Fees | 15,450 | 2,575 | ||||||
| All Other Fees | - | - | ||||||
| Totals | $ | 267,594 | $ | 189,244 | ||||
AUDIT FEES. Consists of fees billed for professional services rendered for the audit of the Company’s annual consolidated financial statements, review of the Form 10-K, and review of the interim consolidated financial statements included in quarterly reports, and services that are normally provided by the Company’s independent auditors in connection with statutory and regulatory filings or engagements, including registration statements.
AUDIT-RELATED FEES. Consists of fees billed for assurance and related services that are reasonably related to the performance of the audit and or review of the Company’s consolidated financial statements and are not reported under “Audit Fees”, such as audits and reviews in connection with acquisitions.
TAX FEES. Consists of fees billed for professional services for tax compliance, tax advice and tax planning.
ALL OTHER FEES. Consists of fees for products and services other than the services reported above. There were no management consulting services provided in fiscal 2020 or 2019.
61
POLICY ON AUDIT COMMITTEE PRE-APPROVAL OF AUDIT AND PERMISSIBLE NON-AUDIT SERVICES OF INDEPENDENT AUDITORS
The current policy of the directors, acting as the audit committee, is to approve the appointment of the principal auditing firm and any permissible audit-related services. The audit and audit related fees include fees for the annual audit of the financial statements and review of financial statements included in 10Q filings. Fees charged by the auditor were approved by the Board with engagement letters signed by the audit committee chairman.
The Audit Committee is responsible for the pre-approval of audit and permitted non-audit services to be performed by the Company’s independent auditor. The Audit Committee will, on an annual basis, consider and, if appropriate, approve the provision of audit and non-audit services by the auditor. Thereafter, the Audit Committee will, as necessary, consider and, if appropriate, approve the provision of additional audit and non-audit services by the auditor which are not encompassed by the Audit Committee’s annual pre-approval and are not prohibited by law. The Audit Committee has delegated to the Chair of the Audit Committee the authority to pre-approve, on a case-by-case basis, non-audit services to be performed by the auditor. The Audit Committee has approved all audit and permitted non-audit services performed by the auditor for the year ended December 31, 2020.
Vote Required for Approval
The approval of the Auditor Proposal requires the affirmative vote of the holders of a majority of votes cast by the stockholders present in person or represented by proxy and entitled to vote thereon at the annual meeting. Accordingly, abstentions and broker non-votes, if any, will have no effect on the outcome of the Auditor Proposal.
AVALON’S BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT ITS STOCKHOLDERS
VOTE “FOR” THE AUDITOR PROPOSAL.
62
PROPOSAL NO. 3—THE NASDAQ PROPOSAL
Acquisition Shares
Pursuant to the terms of the Purchase Agreement, at the Closing, Avalon will issue an aggregate of 81,000,000 shares of Avalon Common Stock to the Sen Lang Shareholders. The shares of Avalon Common Stock issuable to the Sen Lang Shareholders are referred to as the “Acquisition Shares.” The number of Acquisition Shares issuable is subject to appropriate adjustment upon any pre-Closing stock dividend, distribution, stock split, reclassification, combination or other change with respect to shares of Avalon Common Stock effected by Avalon.
For more information about the Acquisition and the issuance of the Acquisition Shares, please see the sections entitled “The Acquisition” and “The Purchase Agreement—Acquisition Consideration”, and the full text of the Purchase Agreement included as Annex A hereto.
Exchange Shares
In connection with and as a condition to the Closing of the Acquisition, Avalon is required to consummate the Equity Financing. Pursuant to the terms of the Exchange Agreement, the Investor shall have the right, exercisable between the six-month and five year-anniversaries of the respective initial closing and installment closings, to elect to exchange, from time to time, all or part of its then-owned equity ownership of SenlangBio for the Exchange Shares at a fixed exchange price of US$1.21 per share of Avalon Common Stock, which was the market price of the Avalon Common Stock as of the date of the Exchange Agreement under Nasdaq rules, or an aggregate of approximately 25,885,000 shares of Avalon Common Stock (based on the consummation of all of the installments of the Equity Financing and utilizing the conversion rate of US dollars to RMB of 6.3856 as of June 11, 2021). The actual number of shares to be issued under the Exchange Agreement may vary due to changes in the RMB exchange rate at the time of an exchange notice.
For more information about the Equity Financing and the issuance of the Exchange Shares, please see the section entitled “The Equity Financing”.
Approval of the Issuance of the Acquisition Shares and the Exchange Shares
The issuance of the Acquisition Shares and the Exchange Shares is expected to result in an issuance comprising more than 20% of the outstanding common stock of Avalon, or more than 20% of the voting power, in each case outstanding before the issuance. The Avalon board of directors is requesting stockholder approval of Proposal 1 to comply with Nasdaq Listing Rules 5635(a) and 5635(d), as applicable.
Vote Required for Approval
The approval of the Nasdaq Proposal requires the affirmative vote of the holders of a majority of votes cast by the stockholders present in person or represented by proxy and entitled to vote thereon at the annual meeting. Accordingly, abstentions and broker non-votes, if any, will have no effect on the outcome of the Nasdaq Proposal.
The Acquisition is conditioned on the approval of the Nasdaq Proposal.
AVALON’S BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT ITS STOCKHOLDERS VOTE “FOR” THE ISSUANCE OF THE ACQUISITION SHARES AND THE EXCHANGE SHARES AS SET FORTH IN THE NASDAQ PROPOSAL.
63
PROPOSAL NO. 4—THE ADJOURNMENT PROPOSAL
If Avalon fails to receive a sufficient number of votes to approve the Nasdaq Proposal, Avalon may propose to adjourn the Avalon annual meeting for the purpose of soliciting additional proxies to approve the Nasdaq Proposal. Avalon currently does not intend to propose adjournment at the Avalon annual meeting if there are sufficient votes to approve the Nasdaq Proposal.
If on the date of the Avalon annual meeting, or a date preceding the date on which the Avalon annual meeting is scheduled, Avalon reasonably believes that (i) it will not receive proxies sufficient to obtain the required vote to approve Proposal 1, whether or not a quorum would be present or (ii) it will not have sufficient shares of Avalon Common Stock represented (whether in person or by proxy) to constitute a quorum necessary to conduct the business of the Avalon annual meeting, Avalon may postpone or adjourn, or make one or more successive postponements or adjournments of, the Avalon annual meeting as long as the date of the Avalon annual meeting is not postponed or adjourned more than an aggregate of 30 calendar days in connection with any postponements or adjournments.
Vote Required for Approval
The approval of the Adjournment Proposal requires the affirmative vote of the holders of a majority of votes cast by the stockholders present in person or represented by proxy and entitled to vote thereon at the annual meeting. Accordingly, abstentions and broker non-votes, if any, will have no effect on the outcome of the Adjournment Proposal.
Adoption of the Adjournment Proposal is not conditioned upon the adoption of any of the other proposals.
AVALON’S BOARD OF DIRECTORS UNANIMOUSLY RECOMMENDS THAT ITS STOCKHOLDERS
VOTE “FOR” THE APPROVAL OF THE ADJOURNMENT PROPOSAL.
64
Overview
SenlangBio is a clinical-stage biotechnology company that focuses on three advanced technology platforms—CAR T-cells, CAR γδT-cells and armTILs—to develop a robust pipeline of innovative and transformative cellular immunotherapies for cancer patients. Chimeric antigen receptor (CAR) T-cells (CAR-T) are natural cell-killing T-cells that have been engineered to specifically recognize and kill cancerous cells. Allogeneic (universal) CAR γδT-cells (CAR-GDT) are a specific class of donor-derived T-cells that can provide superior anti-tumor effectiveness. Armored tumor infiltrating lymphocytes (armTILs) are cancer-killing T-cells that provide a unique “personalized” cellular immunotherapy approach.
SenlangBio is currently the largest cell therapy company in Northern China in terms of the scale of bio-manufacturing, as well as the breadth and depth of active pre-clinical research and clinical development programs.
SenlangBio is currently gathering clinical trial data through in-human “investigator-initiated” clinical trials, which are conducted by SenlangBio in cooperation with several hospitals in China. To date, over 300 patients have received 15 of SenlangBio’s cellular immunotherapies through these early-stage clinical studies at 13 hospitals and medical centers, covering 9 medical indications. Such clinical trials have been designed to fully comply with the same requirements applicable to clinical trials registered with China’s National Medical Product Administration (NMPA), in particular the requirements related to manufacturing quality control, informed consent, data integrity, data management, and GCP requirements.
SenlangBio was formed in 2016 in Hebei Province, China. The principal executive offices of SenlangBio are located at Room 512 and 513, Building 1, 136 Yellow River Avenue, Shijiazhuang High-tech Development Zone, Hebei Province, China, and its telephone number is +86-311-82970975.
Drug Candidate Pipeline
Candidates in the Clinical (Investigator Initiated) Development Stage:
| ● | Senl_1904B: Autologous anti-CD19 CAR-T for relapsed or refractory (R/R) B-cell lymphoblastic leukemia (B-ALL); | |
| ● | Senl_B19: Autologous anti-CD19 CAR-T for R/R non-Hodgkin’s lymphoma (NHL); | |
| ● | Senl_H19: Autologous anti-CD19 CAR-T for post CAR-T relapsed B-ALL and NHL; | |
| ● | Senl_NS7CAR: Autologous anti-CD7 CAR-T for T-cell ALL and T-cell lymphoblastic lymphoma (T-LBL); | |
| ● | Senl_H19x22P: Autologous, dual Anti-CD19 and anti-CD22 CAR-T for R/R B-ALL and NHL |
Candidates in the Preclinical/IND Enabling Stage:
| ● | Senl_ABUCAR7: Universal anti-CD7 CAR-T for R/R T-ALL and T-LBL; | |
| ● | Senl_GDUCARxxx (undisclosed target): Universal CAR-GDT for R/R acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS); | |
| ● | Senl_GDSTCLDxx (undisclosed target): Universal CAR-GDT for certain types of solid tumors; | |
| ● | Senl_BCMA03: Autologous, anti-BCMA CAR-T for R/R multiple myeloma (MM); | |
| ● | Senl_comboCARs (Senl_TAAx22P): Autologous, combo/customized anti-tumor associated antigen (TAA) and anti-CD22/PD-L1 CAR-T for sarcomas, ovarian cancer and other solid tumors; | |
| ● | Senl_armTILs: Autologous, “armored” tumor infiltrating lymphocytes for solid tumors |
65
CAR-T Cell Therapy Platform
SenlangBio’s CAR-T cell therapy portfolio consists of multiple autologous and allogeneic (“off-the-shelf”) candidates with single targets as well as “cocktail” combinations. Currently, SenlangBio’s autologous CAR-T candidates have demonstrated positive remission rates in early clinical studies against R/R B-ALL, T-cell ALL (T-ALL), NHL and T-LBL with a well-tolerated adverse effect profile.
Lead Clinical Candidates in Development
Senl_1904B, SenlangBio’s lead clinical stage candidate, is an autologous anti-CD19 CAR-T, in development for R/R B-ALL and NHL. This CAR-T design has the potential to reduce the risk of cytokine release syndrome (CRS and CAR-related encephalopathy syndrome (CRES) frequent with the current first generation of CAR-T therapies yet retains robust anti-leukemia/lymphoma activities. Senl_1904B has demonstrated a 97.2% (35/36) complete remission rate and only a 5.6% (2/36) Grade 3 CRS among R/R B-ALL and NHL patients in a successfully completed principal investigator (PI)-initiated, open-label, first-in-human clinical trial. SenlangBio has recently received approval by the Chinese Center for Drug Evaluation (CDE), a division of the NMPA, of the company’s Investigational New Drug (IND) application to initiate a new Phase I clinical trial in R/R B-ALL during 3Q2021.
Senl_NS7CAR is an autologous anti-CD7 CAR-T candidate in development for R/R T-ALL and T-LBL. A successfully completed PI-initiated, open-label, first-in-human clinical trial demonstrated that 8 of 8 T-ALL and T-LBL patients achieved complete remission and none developed greater than Grade 2 CRS. SenlangBio plans to file an IND application with the CDE during 3Q2021 and will start an official Phase I clinical trial upon approval of the IND application.
Senl_H19x22P is an autologous dual CAR-T candidate at the clinical development stage for R/R B-ALL and NHL. This CAR-T cell therapy candidate contains a cocktail of H19 CAR modified T cells and 22P CAR modified T cells. Generally, CAR-T cell therapy targeting CD19 has demonstrated promising success rates, however, a high rate of disease relapse remains a hurdle to overcome. An investigator-initiated trial demonstrated technical feasibility, high activity and low toxicities of the Senl_H19x22P CAR-T cell cocktail in treating B-ALL patients who relapsed after treatment with a CD19 CAR-T therapy containing murine single-chain variable fragment (scFv) (FMC63). So far, in a cohort of 7 patients who relapsed after FMC63-CAR therapy and were subsequently infused in an open-label trial with Senl_H19x22P CAR-T cocktails, 6 of them (85.7%) achieved MRD-negative complete remission (CR) within the first month of cell infusion. No patients developed CAR-T related serious (greater than grade-2) toxicities of CRS or CRES. SenlangBio plans to file an IND application with the CDE during 4Q2021 and will start an official Phase I clinical trial upon approval of the IND application.
Select Candidates in Preclinical Development
CAR-gdT cell-therapy candidates:
| ● | Universal/allogeneic (“off-the-shelf”) cell therapy modality | |
| ● | Potentially breakthrough treatment for relapsed and refractory acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) | |
| ● | Targeting multiple solid tumor malignancies, including pancreatic, gastric, ovarian, sarcomas, and others | |
| ● | Successfully completed pre-clinical, IND-enabling stage and currently in preparation to initiate first-in-human clinical trials |
66
Senl_comboCARs are autologous CAR-T candidates customized in design with a combination of anti-tumor associated antigen (anti-TAA) CAR and anti-CD22/PD-L1 CAR. The gene construct is designed to simultaneously express two different CAR structures. The scFv of the first CAR structure can potentially recognize specific tumor-associated antigens, and the scFv of the second CAR structure can potentially target CD22. SenlangBio believes this strategy can enhance the proliferation of CAR-T cells in peripheral blood to ensure more CAR-T cells reaching the tumor locations. The CAR structure targeted to the tumor cell surface is designed to kill tumor cells. Additionally, the combinatorial CAR structure also includes a soluble scFv derived from an anti-PD-L1 monoclonal antibody, which is designed to overcome immunosuppression by blocking the PD-1/PD-L1 pathway. Based on the expression of different tumor associated antigens, scFv (TAA) can potentially be customized and replaced to target individual solid tumors. Currently, SenlangBio has designed and developed a series of comboCAR constructs consisting of individual and/or combinations of tumor-associated antigens which may potentially overcome the current shortage and limitation of CAR-T cell therapies for treatment of solid tumors.
CAR-GDT (CAR-γδT) Cell Therapy Platform
Universal/allogeneic (“off-the-shelf”) cell therapy modality and potentially breakthough therapy for difficult to treat R/R acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). Therapeutic candidates are also targeting difficult to treat solid tumor malignancies, including pancreatic, gastric, and ovarian cancers as well as sarcomas, all of which represent a major unmet need among oncology patients. Several of the candidates have successfully completed the pre-clinical, IND-enabling stage, and SenlangBio is currently in preparation to initiate 3-4 PI-initiated first-in-human clinical trials targeting AML, MDS and solid tumors in 4Q2021.
SenlangBio Clinical Laboratory Business
SenlangBio Clinical Laboratory, a wholly owned subsidiary of SenlangBio that provides third-party clinical testing services, including 1) general biochemical, genomic and proteomic testing; and 2) cell therapy related testing such as hematology, immunology, cancer biomarkers, immuno-phenotyping, and others.
Strengths
By leveraging unique core technology platforms and rapid research-to-clinical translation capabilities, SenlangBio seeks to develop a profile of cell therapy candidates with the following features of differentiating competence:
| ● | Diverse pipeline (“autologous” + “universal”) candidates with potentially potent and durable cancer-killing activities (hematologic + solid tumor malignancies) | |
| ● | High tolerability and established safety profile in clinical trials to date | |
| ● | Provide therapies for novel oncogenic indications that are unmet by the current generation of cellular immunotherapies | |
| ● | Accelerated research-to-clinical translation potentially enabling widespread patient accessibility and broader commercial adoption |
SenlangBio Management
Jianqiang Li, Ph.D., Scientific Founder, CSO, Director
Jianqiang Li has over 20 years of academic and industry experience in immunology and cell therapy. Dr. Li has published 13 papers in top-ranked journals, including Nature Medicine, Blood, and the Journal of Immunology, with a cumulative impact index over 60. Dr. Li is an invited speaker at ASH, ASBMT, ICBS, ECI and Gamma-Delta T Cell International Conference. Dr. Li independently set up an efficient CAR-T production system, achieved major breakthroughs in the development and clinical application of allogeneic CAR γδT-cell therapy, and made important contributions to the antigen presentation and activation mechanism of Vγ9Vδ2 T-cells. He has successfully developed CAR-γδ T-cell products derived from human cord blood. Dr. Li has a Master of Immunology from the Institute of Basic Medicine, Peking Union Medical College (2003-2006), a Doctor of Immunology from the University of Würzburg (2006-2009), was a Postdoctoral Fellow at the City of Hope National Medical Center (2010) and was a Senior Scientist in the R&D Department at Fred Hutchinson Cancer Research Center (2011-2016), before founding SenlangBio.
67
Shengmin Guo, Co-founder, CEO, Director
Shengmin Guo has over 20 years of experience in the pharmaceutical industry. Previously, Ms. Guo worked at CSPC NBP Pharmaceutical Co. Ltd (HKEX: 01093), accumulating 12 years of marketing and management experience as Marketing Director, Deputy Manager and Vice President, and 10 years as Senior Manager of Human Resources. Ms. Guo led the development and commercialization of a novel drug launch.
Facilities and Manufacturing
SenlangBio’s main scientific facility covers a total area of around 5,000m2. Among which, roughly, (1) 1,600m2 is used for production of T-cells, which are solely used for the purpose of clinical trials, (2) 800m2 is used for R&D activities, (3) 800m2 is used for testing services (by SenlangBio Clinical Laboratory), (4) 800m2 is used for office use and (5) 800m2 is used for storage and other purposes.
SenlangBio’s GMP bio-manufacturing facility has the capacity and capability to produce:
| ● | 5 autologous CAR-T production lines with an estimated annual output of 5,000 unit doses; and | |
| ● | 2 universal CAR-γδT production lines with an estimated annual output of 10,000 unit doses. |
Suppliers and Raw Materials
SenlangBio has in-house research and production capabilities for lentiviral vectors, plasmids, T-cell cultures, validation bio-assays, as well as QA/QC processes. Raw materials and supplies are related to basic molecular biology and cell culture reagents and materials, which are generally and readily available globally. Raw materials and supplies used by SenlangBio Clinical Laboratory are also generally and readily available globally.
Competition
The competitive landscape includes companies that are engaging in cell and gene therapies. Considering the CAR-T field, some notable competitors (in similar size and scale of business) include (but not limited to) Gracell Biotechnologies (NASDAQ: GRCL), Legend Biotech Corporation (NASDAQ: LEGN), and Poseida Therapeutics, Inc. (NASDAQ: PSTX). For CAR-gdT cell therapy field, some notable companies include (but not limited to) Adicet Bio Inc. (NASDAQ: ACET), Incysus Therapeutics, Inc and GammaDelta Therapeutics.
SenlangBio’s Advantages over Competitors
SenlangBio provides a total solution approach in the CellTech industry sector with the seamless integration of innovative research, process development, standardization, validation, quality assessment/quality control, bio-manufacturing and clinical development, and therefore aims to accelerate the translation of technology to clinical application as well as lower overall manufacturing costs.
SenlangBio possesses a diverse and broad repertoire of pipeline candidates covering solid and hematologic malignancies; including both autologous and universal (“off-the-shelf”) cell therapy candidates.
SenlangBio’s proprietary in vitro expansion protocol for gamma-delta T cells is a critical technology breakthrough with an over 5,000-fold increase in yield, enabling the industrial scale generation of CAR-GDT cells for clinical development.
SenlangBio has applied its promoter technology to regulate the cell surface density of CAR molecules, leading to the reduction of therapy-related toxicities. Seng_1904B, SenlangBio’s lead cell-therapy candidate for relapsed/refractory B-cell acute lymphoblastic leukemia, is driven by the MND promoter, which has demonstrated a superior safety profile in first-in-human investigator initiated clinical trials.
While conventional CAR-T therapy is limited to B-cell malignancies, SenlangBio’s technology platform targets the full spectrum of blood cancers, including both B-cell and T-cell malignancies. SenlangBio’s Seng_NS7CAR candidate has demonstrated positive activities in first in first-in-human investigator initiated clinical trials for treatment of relapsed/refractory T-cell lymphoblastic leukemia (T-ALL) and T-cell acute lymphoblastic lymphoma (T-LBL).
68
Employees
As of June 30, 2021, SenlangBio had 128 employees, among which 104 employees work for SenlangBio and 24 employees work for SenlangBio Clinical Laboratory.
Government Regulation
Currently, all of SenlangBio’s development work is being conducted in China. China has a dual-track regulatory pathway for conducting T-cell therapy clinical trials. The first track is approval for a health care-affiliated clinical study managed by the National Health Commission, China’s primary healthcare regulatory agency (the “NHC”) (the “IIT Pathway”). The other pathway is to register as a biological drug which requires an IND, a registered clinical trial and NDA approval by the Center for Drug Evaluation (CDE), a subdivision of China’s National Medical Product Administration (NMPA) prior to commercialization (the “Drug Pathway”).
| 1. | IIT Pathway |
IIT stands for investigator-initiated clinical trials, where such trials are initiated and conducted under the oversight of the NHC as a medical practice technology, rather than the NMPA as a medical product. The NMPA, generally speaking, will accept, review, and reject or approve an IND application only from the manufacturer of the investigational product as the sponsor of the IND, rather than from a physician who intends to be the investigator and sponsor of the IND. The NMPA distinguishes the former as a registered clinical trial (namely the clinical trial introduced in the section of “Drug Pathway”), and the latter as a non-registered clinical trial, and normally will not consider the data generated from investigator-initiated, non-registered clinical trials when it reviews the application for a registered clinical trial from the manufacturer.
In the case of CAR-T therapy, however, the NMPA is aware of the large number of investigator-initiated non-registered clinical trials in China and the United States, and some reviewers from the CDE have published two articles on its website in February 2018 and October 2018 expressing the view that (1) the mainstream regulatory oversight is to follow the pathway of registered clinical trials, but that (2) data from investigator-initiated non-registered clinical trials may be considered if the non-registered clinical trials otherwise fully comply with the same requirements applicable to registered clinical trials, in particular the requirements related to manufacturing quality control, informed consent, data integrity, data management, and all GCP requirements.
In March 2019, a Draft Somatic Cell Therapy Clinical Research and the Transformation Application Management Measures was released by the NHC, which stipulated, among others, that after filing with the NHC, hospitals may use somatic cell therapy treatment and charge patients. However, so far such measures do not come into effect, and any medical institutions or biotech companies which choose the IIT Pathway to conduct T-cell therapy shall be regulated as a clinical study within medical institutions and the IIT Pathway shall not be utilized for the purpose of commercialization.
In addition, sponsors taking the IIT Pathway need to complete the filing of the IIT with the competent NHC branch and obtain ethics review approval for the related clinical trials.
| 2. | Drug Pathway |
Pursuant to PRC law, human cell therapy and its products are categorized as biological products and the application for biological products shall be submitted as part of the process of a new drug application.
On November 30, 2017, the NMPA promulgated the Notice of Guidelines for Acceptance and Examination of Drug Registration (Trial), which provides that the application for clinical trials of therapeutic biological products and the production and listing application for therapeutic biological products shall be subject to the provisions thereof. On December 18, 2017, the NMPA promulgated the Technical Guiding Principles for Research and Evaluation of Cell Therapy Products (Trial), which sets out the guidelines for medical study, non-clinical research and clinical research of cell therapy products.
As for the medical study of cell therapy, the general principle is that the medical studies and quality control of cell therapy shall take into account the fact that cells are capable of living in a body, multiplying and/or differentiating. At the same time, cell therapy products should meet the general requirements of drug quality management, and the whole production process of clinical samples should meet the basic principles and relevant requirements of the Good Manufacturing Practice for Drugs, or the GMP Regulations, published by the Ministry of Health on December 28, 1992 and further amended on January 17, 2011.
69
According to the Technical Guiding Principles for Cell Therapy Products, non-clinical research shall follow the following principles:
| (a) | the research and evaluation of different products should follow the principle of a “case by case analysis” while at the same time, the Guidelines for the Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals issued by the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use should serve as a reference for the evaluation of non-clinical research of cell therapy products; | |
| (b) | non-clinical study evaluation trials should use cell therapy products intended for clinical trials whenever possible. The production process and quality control of a subject used in a non-clinical trial shall be consistent with that of the subject to be used in a clinical trial (if not, the subject shall be explained and its effect on the prediction of human response shall be assessed); | |
| (c) | non-clinical study evaluation should be conducted by selecting suitable species of animals whose biological response to cell therapy products is close to or similar to the expected human response. In some cases, alternatives to animal sources may also be used for evaluation; | |
| (d) | in non-clinical study evaluation, the administration of cell therapy products should be able to maximize the simulation of the proposed clinical administration. If clinical administration cannot be simulated in animal studies, alternative administration methods should be identified in pre-clinical studies and their scientific and rational nature should be clarified; and | |
| (e) | subject analysis data should be provided. |
As for clinical trials, the Technical Guiding Principles for Cell Therapy Products stipulate that when cell therapy products enter clinical trials, they should follow the requirements of the GCP. In principle, the research contents of clinical trials should include a clinical safety evaluation, pharmacokinetics study, pharmacodynamics study, dose exploration study and confirmatory clinical trials. According to the product nature of different cell therapy products, the specific test design can be adjusted as appropriate.
On 13 March 2018, the CDE promulgated the Key Considerations in Applying for Clinical Trials of Cell Therapy Products for Pharmaceutical Research and Application Data to encourage the innovation of cell therapy products in view of the urgent need of such drugs. The document provides guidance on the preparation of pharmaceutical research and application materials in the application phase of clinical trials, according to which, on the basis of following the requirements of technical guidelines for carrying out relevant research, the applicant should pay special attention to certain considerations of pharmaceutical research and application materials, including production of raw materials, production process, quality studies, and stability studies. On the basis of the Technical Guiding Principles for Cell Therapy Products, on 18 October 2019, the CDE promulgated the Pharmaceutical Research Questions and Answers for Application of Cell Therapy Products for Clinical Trials (Issue One) to provide further reference for applicants on the common problems in the review and communication of IND application data of cell therapy products. On February 10, 2021, the CDE published the Technical Guidelines for Clinical Trials of Immunocell Therapy Products, the guidelines provide necessary technical guidance for the overall planning, design, implementation, and data analysis of cellular immune treatment (including CAR-T) products to carry out clinical trials, to reduce certain risks of the participating subjects in clinical trials and to regulate the evaluation method of the safety and effectiveness of such treatment. The guidelines are not mandatory.
Given the uniqueness of CAR-T and human cell therapies and that the regulatory pathway for such therapies is still evolving, standardization for CAR-T therapies is difficult to achieve and therefore approval would be assessed on a case-by-case basis.
Intellectual Property
SenlangBio currently protects its intellectual property and commercial exclusivity through a combination of Chinese patents, trade secrets, trademarks and copyright. SenlangBio currently owns a total of ten Chinese patents (including two inventions and eight utility models) and five Chinese patents applications (including three invention applications and two utility model applications), as well as eight software copyrights and two trademarks. What follows is a summary of relevant intellectual property laws and principles in China.
70
| (1) | Patents |
Pursuant to the PRC Patent Law, most recently amended in October 2020 (the amendment is implemented on June 1, 2021), and its implementation rules, most recently amended in January 2010, patents in China fall into three categories: invention, utility model and design. An invention patent is granted to a new technical solution proposed in respect of a product or method or an improvement of a product or method. A utility model is granted to a new technical solution that is practicable for application and proposed in respect of the shape, structure (or a combination of both) of a product. A design patent is granted to a new design of a certain product in shape, pattern (or a combination of both) and in color, shape and pattern combinations aesthetically suitable for industrial application. Under the PRC Patent Law, the term of patent protection starts from the date of application. Patents relating to invention are effective for twenty years, and utility model and design patents are effective for ten years from the date of application. The PRC Patent Law adopts the principle of “first-to-file” system, which provides that where more than one person files a patent application for the same invention, a patent will be granted to the person who first files the application.
Existing patents can be narrowed, invalidated or unenforceable due to a variety of grounds, including lack of novelty, creativity, and deficiencies in patent application. In China, a patent must have novelty, creativity and practical applicability. Under the PRC Patent Law, novelty means that before a patent application is filed, no identical invention or utility model has been publicly disclosed in any publication in China or overseas or has been publicly used or made known to the public by any other means, whether in or outside of China, nor has any other person filed with the patent authority an application that describes an identical invention or utility model and is recorded in patent application documents or patent documents published after the filing date. Creativity means that, compared with existing technology, an invention has prominent substantial features and represents notable progress, and a utility model has substantial features and represents any progress. Practical applicability means an invention or utility model can be manufactured or used and may produce positive results. Patents in China are filed with the State Intellectual Property Office, or SIPO. Normally, the SIPO publishes an application for an invention patent within 18 months after the filing date, which may be shortened at the request of applicant. The applicant must apply to the SIPO for a substantive examination within three years from the date of application.
The PRC Patent Law provides that, for an invention or utility model completed in China, any applicant (not limited to Chinese companies and individuals), before filing a patent application outside of China, must first submit it to the SIPO for a confidential examination. Failure to comply with this requirement will result in the denial of any Chinese patent for the relevant invention. This added requirement of confidential examination by the SIPO has raised concerns by foreign companies that conduct research and development activities in China or outsource research and development activities to service providers in China.
| (2) | Trade Secrets |
According to the PRC Anti-Unfair Competition Law, the term “trade secrets” refers to technical and business information that is unknown to the public, has utility and may create business interests or profits for its legal owners or holders, and is maintained as a secret by its legal owners or holders. Trade secret requirements under the current framework in China is still under development and not robust.
Under the PRC Anti-Unfair Competition Law, which was promulgated on September 2, 1993 and was latest amended on April 23, 2019, business persons are prohibited from infringing others’ trade secrets by: (1) acquiring a trade secret from the right holder by theft, bribery, fraud, coercion, electronic intrusion, or any other illicit means; (2) disclosing, using, or allowing another person to use a trade secret acquired from the right holder by any means as specified in the item (1) above; (3) disclosing, using, or allowing another person to use a trade secret in its possession, in violation of its confidentiality obligation or the requirements of the right holder for keeping the trade secret confidential; (4) abetting a person, or tempting, or aiding a person into or in acquiring, disclosing, using, or allowing another person to use the trade secret of the right holder in violation of his or her non-disclosure obligation or the requirements of the right holder for keeping the trade secret confidential. If a third party knows or should have known that an employee or former employee of the right owner of trade secrets or any other entity or individual conducts any of the illegal acts listed above, but still accepts, publishes, uses or allows any other to use such secrets, this practice will be deemed as an infringement of trade secrets. A party whose trade secrets are being misappropriated may petition for administrative corrections, and regulatory authorities may stop any illegal activities and fine infringing parties in the amount of RMB100,000 to RMB1,000,000, and where the circumstance is serious, the fine will be RMB500,000 to RMB5,000,000. Alternatively, persons whose trade secrets are being misappropriated may file lawsuits in a Chinese court for loss and damages incurred due to the misappropriation.
71
The measures to protect trade secrets include oral or written non-disclosure agreements or other reasonable measures to require the employees of, or persons in business contact with, legal owners or holders to keep trade secrets confidential. Once the legal owners or holders have asked others to keep trade secrets confidential and have adopted reasonable protection measures, the requested persons bear the responsibility for keeping the trade secrets confidential.
| (3) | Trademarks |
Pursuant to the Trademark Law of the PRC promulgated by the Standing Committee of the NPC on August 23, 1982 and last amended on April 23, 2019, which amendment became effective from November 1, 2019, the period of validity for a registered trademark is ten years, commencing from the date of registration. The registrant shall go through the formalities for renewal within twelve months prior to the expiry date of the trademark if continued use is intended. Where the registrant fails to do so, a grace period of six months may be granted. The validity period for each renewal of registration is ten years commencing from the day immediately after the expiry of the preceding period of validity for the trademark. In the absence of a renewal upon expiry, the registered trademark shall be canceled. Industrial and commercial administrative authorities have the authority to investigate any behavior in infringement of the exclusive right under a registered trademark in accordance with the law. In case of a suspected criminal offense, the case shall be timely referred to a judicial authority and decided according to the law.
| (4) | Copyright |
Pursuant to the Copyright Law of the PRC, effective in June 1, 1991 and most recently amended in November 2020 (the amendment was implemented on June 1, 2021), copyrights include personal rights such as the right of publication and that of attribution as well as property rights such as the rights of production and distribution. Reproducing, distributing, performing, projecting, broadcasting or compiling a work or communicating the same to the public via an information network without permission from the owner of the copyright therein, unless otherwise provided in the Copyright Law of the PRC, constitutes infringements of copyrights. The infringer must, according to the circumstances of the case, undertake to cease the infringement, take remedial action, and offer an apology or pay damages.
Pursuant to the Computer Software Copyright Protection Regulations promulgated on December 20, 2001 and last amended on January 30, 2013, a software copyright owner may complete registration formalities with a software registration authority recognized by the State Council’s copyright administrative department. A software copyright owner may authorize others to exercise that copyright, and is entitled to receive remuneration.
The VIE Structure
Neither Sen Lang nor any of its subsidiaries owns any equity interest in SenlangBio. Instead, it controls and receives the economic benefits of SenlangBio’s business operation through a series of contractual arrangements.
On April 26, 2021, the PRC Subsidiary, which is indirectly wholly-owned by Sen Lang, entered into a series of contractual arrangements, or VIE Agreements, with SenlangBio and the 13 equity holders of SenlangBio, through which Sen Lang obtained control and became the primary beneficiary of SenlangBio, hereinafter referred to as the Reorganization. As a result, SenlangBio became Sen Lang’s “VIE.”
Sen Lang was established as an indirect holding company of the PRC Subsidiary.
72
The following chart illustrates Sen Lang’s corporate structure, including its subsidiaries, consolidated variable interest entity and VIE’s subsidiary as of June 24, 2021 (the issuance date of Sen Lang’s historical financial statements included herein):
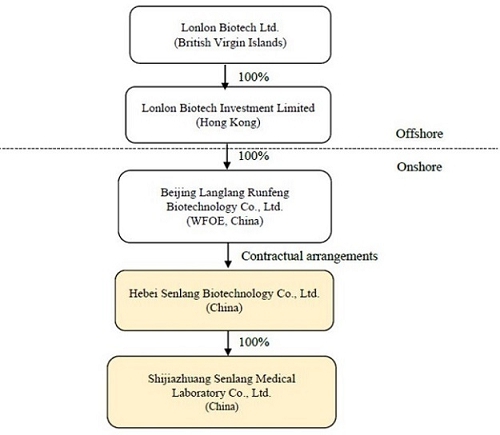
VIE Agreements with SenlangBio
Upon the completion of the Reorganization, Sen Lang, through the PRC Subsidiary, entered into the following contractual arrangements with the VIE and the VIE’s 13 shareholders that enabled Sen Lang to (1) have power to direct the activities that most significantly affects the economic performance of the VIE, and (2) receive the economic benefits of the VIE that could be significant to the VIE. Accordingly, the PRC Subsidiary was considered the primary beneficiary of the VIE and had consolidated the VIE and the VIE’s subsidiary’s financial results of operations, assets and liabilities in Sen Lang’s consolidated financial statements.
Contracts that give Sen Lang effective control of the VIE
Equity Pledge Agreement
Under the Equity Pledge Agreement between the PRC Subsidiary, SenlangBio and SenlangBio’s shareholders, SenlangBio’s shareholders agree to pledge all of their equity interests in SenlangBio to the PRC Subsidiary to guarantee the performance of SenlangBio and SenlangBio’s shareholders’ obligations under the Exclusive Technical Consultation and Service Agreement, the Exclusive Purchase Option Agreement, the Shareholder’s Rights Proxy Agreement, and the Spousal Consent (“Transaction Agreements”). Under the terms of the Equity Pledge Agreement, in the event that SenlangBio or SenlangBio’s shareholders breach their respective contractual obligations under the Transaction Agreements or the Equity Pledge Agreement, the PRC Subsidiary, as pledgee, is entitled to directly exercise the pledge right and to notify SenlangBio’s shareholders to immediately repay or pay the loans or other payables under the Transaction Agreements. SenlangBio’s shareholders further agreed not to dispose of the pledged equity interests without prior written consent from the PRC Subsidiary.
73
The pledge is effective on the date when the registration of the equity pledge is completed with the competent administration for industry and commerce, and the term of validity of the pledge is the same as the longest term of validity in the Transaction Agreements.
The purposes of the Equity Pledge Agreement are to (1) guarantee the performance of SenlangBio and SenlangBio’s shareholders’ obligations under the Transaction Agreements, (2) make sure SenlangBio’s shareholders do not transfer or assign the pledged equity interests, or create or allow any encumbrance that would prejudice interests of the PRC Subsidiary without prior written consent from the PRC Subsidiary, and (3) provide the PRC Subsidiary control over SenlangBio.
In the event SenlangBio or SenlangBio’s shareholders breaches their contractual obligations under the Transaction Agreements or Equity Pledge Agreement, the PRC Subsidiary will be entitled to (1) be compensated on a preferential basis with the proceeds from the conversion, auction or sale of the pledged equity, and (2) notify SenlangBio’s shareholders to immediately repay the loans or other payables under the Transaction Agreements.
Exclusive Purchase Option Agreement
Under the Exclusive Purchase Option Agreement, SenlangBio’s shareholders irrevocably grants the PRC Subsidiary (or its designee) an exclusive right to purchase the equity held by SenlangBio’s shareholders in the SenlangBio in whole or in part at any time during the term of the agreement; SenlangBio also irrevocably grants the PRC Subsidiary (or its designee) an exclusive right to purchase the assets owned by SenlangBio in whole or in part at any time during the term of the agreement.
With respect to consideration for equity purchase, the PRC Subsidiary has the right to purchase all or part of the equity held by SenlangBio’s shareholders in SenlangBio at the lowest price permitted by the PRC laws. With respect to the price for asset purchase, the PRC Subsidiary has the right to purchase SenlangBio’s assets at a price equivalent of the net book value of the purchased assets; provided that if the minimum price permitted by the PRC law is higher than the net book value, the minimum price permitted by the PRC laws will prevail.
Under the Exclusive Purchase Option Agreement, the PRC Subsidiary may purchase all or part of the equity held by SenlangBio’s shareholders in SenlangBio and all or part of the assets owned by SenlangBio at any time to the extent permitted by PRC laws. The Exclusive Purchase Option Agreement, together with the Equity Pledge Agreement, Exclusive Technical Consultation and Service Agreement, and the Proxy Agreement, enable the PRC Subsidiary to exercise effective control over SenlangBio. The Exclusive Purchase Option Agreement remains in effect, unless terminated by the PRC Subsidiary by giving a thirty (30) day advance written notice.
Shareholder’s Rights Proxy Agreement
Under the Shareholder’s Rights Proxy Agreement, SenlangBio’s shareholders authorize any entity or individual designated by the PRC Subsidiary to act on their behalf as their exclusive agent to exercise all shareholder’s rights under the PRC laws and SenlangBio’s Articles of Association, including but not limited to: (a) to convene, attend and vote on all the matters during shareholders’ meetings; (b) to transfer, pledge or dispose of, or create encumbrance on the equity; (c) to receive dividends; (d) to participate in judicial proceedings or execute legal documents in relation to shareholders’ rights; (e) to appoint legal representatives, directors and officers of SenlangBio; and (f) to enter into contracts and exercise the Exclusive Purchase Option Agreement.
The Shareholder’s Rights Proxy Agreement shall remain effective until the earlier of: (a) the date on which SenlangBio’s shareholders are no longer the nominee or actual shareholders of the PRC Subsidiary; (b) the date on which the PRC Subsidiary requests the proxy to be terminated in writing; or (c) the date on which the assets and licenses of SenlangBio have been fully transferred to the PRC Subsidiary.
74
Contracts that enable Sen Lang to receive substantially all of the economic benefits from the VIE
Exclusive Technical Consultation and Service Agreement
Pursuant to the Exclusive Technical Consultation and Service Agreement between the PRC Subsidiary and SenlangBio, the PRC Subsidiary provides SenlangBio with technical consultation and services, including conducting market research, assisting with developing management and sales plans, implementing relevant technology application, and providing other consultation services for computer network, finance, business, legal affairs, operation, human resources, and other aspects of SenlangBio. Additionally, the PRC Subsidiary agrees to grant SenlangBio its trademarks, software copyrights, management systems, management methods and other intellectual property in relation to its services on a chargeable and revocable basis, but such grant shall not result in the transfer of any intellectual property or create any restriction on the PRC Subsidiary’s full ownership.
For services rendered to SenlangBio under this agreement, the PRC Subsidiary is entitled to collect a service fee calculated based on the complexity of services rendered, time required by the PRC Subsidiary, and the exact contents and commercial value of services rendered. During the term of this agreement, the PRC Subsidiary shall enjoy all the economic benefits derived from SenlangBio’s operations, and in the event of serious difficulties in SenlangBio’s operations, the PRC Subsidiary may provide SenlangBio with financial support, and the PRC Subsidiary has the right to request SenlangBio to cease operation. The Exclusive Technical Consultation and Service Agreement shall remain in effect for ten (10) years and shall be automatically renewed unless it is terminated earlier by the PRC Subsidiary.
Based on the foregoing VIE Agreements, the PRC Subsidiary has effective control of SenlangBio which enables the PRC Subsidiary to receive all of the expected residual returns and absorb the expected losses of the VIE and its subsidiary. Sen Lang’s management therefore concluded that Sen Lang, through the above contractual arrangements, has the power to direct the activities that most significantly impact the VIE’s economic performance, bears the risks of and enjoys the rewards normally associated with ownership of the VIE, and therefore Sen Lang is the ultimate primary beneficiary of the VIE. Consequently, Sen Lang consolidates the accounts of SenlangBio and its subsidiary for the periods presented in the financial statements of Sen Lang included in this proxy statement, in accordance with Accounting Standards Codification (“ASC”) 810-10, Consolidation.
Legal Matters
SenlangBio is not currently subject to any material legal proceedings; however, SenlangBio may from time to time become a party to various legal proceedings arising in the ordinary course of its business.
75
SEN LANG MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of financial condition and results of operations should be read together with Sen Lang’s financial statements and related notes included elsewhere in this proxy statement. This discussion of Sen Lang’s financial condition and results of operations contains certain statements that are not strictly historical and are “forward-looking” statements and involve a high degree of risk and uncertainty. Actual results may differ materially from those projected in the forward-looking statements due to other risks and uncertainties that exist in Sen Lang’s operations, development efforts and business environment, including those set forth in the section titled “Risk Factors” in this proxy statement and the other risks and uncertainties described elsewhere in this proxy statement. All forward-looking statements included in this proxy statement involve risks and uncertainties, are based on information available to Sen Lang as of the date indicated, and Sen Lang assumes no obligation to update any such forward-looking statement. All references in this section to “Sen Lang,” the “Company,” “we,” “us,” or “our” mean Lonlon Biotech Ltd., unless we state otherwise, or the context otherwise indicates.
Impact of COVID-19 on our Operations, Financial Condition, Liquidity and Results of Operations
The ultimate impact of the COVID-19 pandemic on our operations is unknown and will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration of the COVID-19 outbreak, new information which may emerge concerning the severity of the COVID-19 pandemic, and any additional preventative and protective actions that governments, or us, may determine are needed.
The occurrence of an uncontrollable event such as the COVID-19 pandemic could negatively impact our operations even though the pandemic did not significantly impact our operation in 2020. However, given the dynamic nature of these circumstances, the uncertainty around the potential resurgence of the COVID-19 cases in China, and the instability of local policies and restrictions, the COVID-19 impact over our business in the year of 2021 cannot be reasonably estimated at this time. If COVID-19 cases resurged in the area we conducted our business and local government implemented new restrictions in the effort to contain the spread, it is expected our business will be negatively impacted.
Overview
Lonlon Biotech Ltd. (“Sen Lang” or the “Company”) is a clinical-stage biotechnology company that focuses on three advanced technology platforms—CAR T-cells, CAR γδT-cells and armTILs—to develop a robust pipeline of innovative and transformative cellular immunotherapies for cancer patients. Chimeric antigen receptor (CAR) T-cells (CAR-T) are natural cell-killing T-cells that have been engineered to specifically recognize and kill cancerous cells. Allogeneic (universal) CAR γδT-cells (CAR-GDT) are a specific class of donor-derived T-cells that can provide superior anti-tumor effectiveness. Armored tumor infiltrating lymphocytes (armTILs) are cancer-killing T-cells that provide a unique “personalized” cellular immunotherapy approach.
The Company is currently the largest cell therapy company in Northern China in terms of the scale of bio-manufacturing, as well as the breadth and depth of active pre-clinical research and clinical development programs.
The Company is currently gathering clinical trial data through in-human “investigator-initiated” clinical trials, which are conducted by it in cooperation with several hospitals in China. To date, over 300 patients have received 15 of its cellular immunotherapies through these early-stage clinical studies at 13 hospitals and medical centers, covering 9 medical indications. Such clinical trials have been designed to fully comply with the same requirements applicable to clinical trials registered with China’s National Medical Product Administration (NMPA), in particular the requirements related to manufacturing quality control, informed consent, data integrity, data management, and GCP requirements.
The Company also provides third-party clinical testing services, including 1) general biochemical, genomic and proteomic testing; and 2) cell therapy related testing such as hematology, immunology, cancer biomarkers, immuno-phenotyping, and others.
76
The conversion of RMB into foreign currencies such as the U.S. dollar (“USD”) have generally been based on rates set by the People’s Bank of China, which are set daily based on the previous day’s interbank foreign exchange market rates and current exchange rates on the world financial markets.
Going Concern and Liquidity and Other Uncertainties
The Company has a limited operating history and its continued growth is dependent upon the continuation of providing general laboratory testing and immunology and hematology testing services, and obtaining additional financing to fund future obligations and pay liabilities arising from normal business operations. In addition, the current cash balance cannot be projected to cover the operating expenses for the next twelve months from the release date of this filing. These matters raise substantial doubt about the Company’s ability to continue as a going concern. The ability of the Company to continue as a going concern is dependent on the Company’s ability to raise additional capital, implement its business plan, and generate significant revenues. There are no assurances that the Company will be successful in its efforts to generate significant revenues, maintain sufficient cash balance or report profitable operations or to continue as a going concern. The Company plans on raising capital through bank and other borrowings to implement its business plan. However, there is no assurance these plans will be realized and that any additional financings will be available to the Company on satisfactory terms and conditions, if any.
Avalon and SenlangBio currently expect that SenlangBio’s cash reserves, based on cash balances at March 31, 2021, together with the proceeds from the Equity Financing, will fund SenlangBio’s operations for 18 months following the closing of the Acquisition, based on current plans and assumptions.
The consolidated financial statements for the year ended December 31, 2020 and the unaudited condensed consolidated financial statements for the three months ended March 31, 2021, included in this proxy statement, were prepared on the basis of a going concern, which contemplates that the Company will be able to realize assets and discharge liabilities in the normal course of business. Accordingly, they do not give effect to adjustments that would be necessary should the Company be required to liquidate its assets. The ability of the Company to meet its obligations, and to continue as a going concern is dependent upon the availability of future funding and the continued growth in revenues. The aforementioned annual and interim consolidated financial statements do not include any adjustments that might result from the outcome of these uncertainties.
Critical Accounting Policies
Use of Estimates and Assumptions
Our discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”). The preparation of these consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. We continually evaluate our estimates, including those related to the useful lives of property and equipment, assumptions used in assessing impairment of long-term assets, and valuation of deferred tax assets and the associated valuation allowances.
We base our estimates on historical experience and on various other assumptions that we believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Any future changes to these estimates and assumptions could cause a material change to our reported amounts of revenues, expenses, assets and liabilities. Actual results may differ from these estimates under different assumptions or conditions.
77
Revenue Recognition
Effective January 1, 2018, the Company began recognizing revenue under Accounting Standards Codification (“ASC”) Topic 606, Revenue from Contracts with Customers (“ASC 606”), using the modified retrospective transition method. The impact of adopting the new revenue standard was not material to the Company’s consolidated financial statements and there was no adjustment to beginning accumulated deficit on January 1, 2018. The core principle of the revenue standard is that a company should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. The following five steps are applied to achieve that core principle:
| ● | Step 1: Identify the contract with the customer |
| ● | Step 2: Identify the performance obligations in the contract |
| ● | Step 3: Determine the transaction price |
| ● | Step 4: Allocate the transaction price to the performance obligations in the contract |
| ● | Step 5: Recognize revenue when the company satisfies a performance obligation |
In order to identify the performance obligations in a contract with a customer, a company must assess the promised goods or services in the contract and identify each promised goods or service that is distinct. A performance obligation meets ASC 606’s definition of a “distinct” goods or service (or bundle of goods or services) if both of the following criteria are met:
| ● | The customer can benefit from the goods or service either on its own or together with other resources that are readily available to the customer (i.e., the goods or service is capable of being distinct). |
| ● | The entity’s promise to transfer the goods or service to the customer is separately identifiable from other promises in the contract (i.e., the promise to transfer the goods or service is distinct within the context of the contract). |
If a goods or service is not distinct, the goods or service is combined with other promised goods or services until a bundle of goods or services is identified that is distinct.
The transaction price is the amount of consideration to which an entity expects to be entitled in exchange for transferring promised goods or services to a customer, excluding amounts collected on behalf of third parties (for example, some sales taxes). The consideration promised in a contract with a customer may include fixed amounts, variable amounts, or both. Variable consideration is included in the transaction price only to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved.
The transaction price is allocated to each performance obligation on a relative standalone selling price basis. The transaction price allocated to each performance obligation is recognized when that performance obligation is satisfied, at a point in time or over time as appropriate.
The Company’s revenues are derived from providing general laboratory testing services and immunology and hematology testing services for patients and other customers. Revenues related to its service offerings are recognized at a point in time when service is rendered. Any payments received in advance of the performance of services are recorded as deferred revenue until such time as the services are performed. Except for deferred revenue, the Company did not have any other contract liability nor contract asset as of March 31, 2021.
The Company does not offer promotional payments, customer coupons, rebates or other cash redemption offers to its customers.
The Company has concluded that its government grants are not within the scope of ASC Topic 606 as they do not meet the definition of a contract with a customer. The Company has concluded that the grants meet the definition of a contribution and are non-reciprocal transactions, and has also concluded that Subtopic 958-605, Not-for-Profit-Entities-Revenue Recognition does not apply, as it is a business entity and the grants are with governmental agencies.
78
In the absence of applicable guidance under U.S. GAAP, effective January 1, 2018, the Company developed a policy for the recognition of grant revenue when the related costs are incurred or the related long-live assets are purchased, and the right to payment is realized.
The Company believes this policy is consistent with the overarching premise in ASC Topic 606, to ensure that revenue recognition reflects the transfer of promised goods or services to customers in an amount that reflects the consideration that it expects to be entitled to in exchange for those goods or services, even though there is no exchange as defined in ASC Topic 606.
The Company believes the recognition of revenue as costs are incurred or long-live assets are purchased, and amounts become realizable is analogous to the concept of transfer of control of a service over time under ASC Topic 606.
Income Taxes
The Company accounts for income taxes using the asset/liability method prescribed by ASC 740, “Income Taxes.” Under this method, deferred tax assets and liabilities are determined based on the difference between the financial reporting and tax bases of assets and liabilities using enacted tax rates that will be in effect in the period in which the differences are expected to reverse. The Company records a valuation allowance to offset deferred tax assets if, based on the weight of available evidence, it is more-likely-than-not that some portion, or all, of the deferred tax assets will not be realized. The effect on deferred taxes of a change in tax rates is recognized as income or loss in the period that includes the enactment date.
The Company follows the accounting guidance for uncertainty in income taxes using the provisions of ASC 740 “Income Taxes”. Using that guidance, tax positions initially need to be recognized in the financial statements when it is more likely than not the position will be sustained upon examination by the tax authorities. As of March 31, 2021, the Company had no significant uncertain tax positions that qualify for either recognition or disclosure in the financial statements. Tax year that remains subject to examination is the years ended December 31, 2017 through December 31, 2020. The Company recognizes interest and penalties related to significant uncertain income tax positions in income tax expense. However, no such interest and penalties were recorded as of March 31, 2021.
Commitment and Contingencies
In the normal course of business, the Company is subject to contingencies, such as legal proceedings and claims arising out of its business, that cover a wide range of matters. Liabilities for such contingencies are recorded when it is probable that a liability has been incurred and the amount of the assessment can be reasonably estimated.
Recent Accounting Standards
In August 2018, the FASB issued ASU No. 2018-13, Fair Value Measurement (Topic 820): Disclosure Framework-Changes to the Disclosure Requirements for Fair Value Measurement. The objective of ASU 2018-13 is to improve the effectiveness of disclosures in the notes to the financial statements by removing, modifying, and adding certain fair value disclosure requirements to facilitate clear communication of the information required by generally accepted accounting principles. The amendments are effective for all entities for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2019 with early adoption permitted upon issuance of this ASU. The adoption of ASU 2018 – 13 did not have a material impact on the Company’s consolidated financial statements.
In June 2016, the FASB issued ASU 2016-13, Financial Instruments - Credit Losses (“Topic 326”). The ASU introduces a new accounting model, the Current Expected Credit Losses model (“CECL”), which requires earlier recognition of credit losses and additional disclosures related to credit risk. The CECL model utilizes a lifetime expected credit loss measurement objective for the recognition of credit losses at the time the financial asset is originated or acquired. ASU 2016-13 is effective for annual period beginning after December 15, 2022, including interim reporting periods within those annual reporting periods. The Company expects that the adoption will not have a material impact on the Company’s consolidated financial statements.
In December 2019, the FASB issued ASU 2019-12, Simplifying the Accounting for Income Taxes, as part of its Simplification Initiative to reduce the cost and complexity in accounting for income taxes. This standard removes certain exceptions related to the approach for intra period tax allocation, the methodology for calculating income taxes in an interim period and the recognition of deferred tax liabilities for outside basis differences. It also amends other aspects of the guidance to help simplify and promote consistent application of GAAP. The guidance is effective for interim and annual periods beginning after December 15, 2020, with early adoption permitted. The adoption of ASU 2019-12 did not have a material impact on the Company’s consolidated financial statements.
79
Other accounting standards that have been issued or proposed by FASB that do not require adoption until a future date are not expected to have a material impact on the consolidated financial statements upon adoption. The Company does not discuss recent pronouncements that are not anticipated to have an impact on or are unrelated to its consolidated financial condition, results of operations, cash flows or disclosures.
RESULTS OF OPERATIONS
Comparison of Results of Operations for the Three Months Ended March 31, 2021 and 2020
Revenues
For the three months ended March 31, 2021, we had general laboratory testing services revenue of $945,648, as compared to $42,887 for the three months ended March 31, 2020, an increase of $902,761, or 2,105.0%. The significant increase was primarily attributable to (i) business expansion; (ii) ongoing boom in COVID testing in Shijiazhuang City, Hebei province. We expect that our revenue from general laboratory testing services will continue to increase in the near future.
For the three months ended March 31, 2021, we had immunology and hematology testing services revenue of $301,857, as compared to $0 for the three months ended March 31, 2020, an increase of $301,857, or 100.0%, resulting from business expansion. We expect that our revenue from immunology and hematology testing services will continue to increase in the near future.
Costs of revenues
Costs of general laboratory testing services include the cost of inventory, labor and related benefits, depreciation, and other overhead costs.
For the three months ended March 31, 2021, costs of general laboratory testing services amounted to $347,911, as compared to $14,117 for the three months ended March 31, 2020, an increase of $333,794, or 2,364.5%. The increase was mainly due to increase in general laboratory testing services revenue.
Costs of immunology and hematology testing services include the cost of inventory, labor and related benefits, depreciation, and other overhead costs.
For the three months ended March 31, 2021, costs of immunology and hematology testing services amounted to $89,498. There were no comparative revenue and related costs of revenue from our immunology and hematology testing services for the three months ended March 31, 2020.
Gross profit
Gross profit from general laboratory testing services for the three months ended March 31, 2021 was $597,737, as compared to $28,770 for the three months ended March 31, 2020, a change of $568,967, or 1,977.6%. Gross margin decreased to 63.2% for the three months ended March 31, 2021 from gross margin of 67.1% for the three months ended March 31, 2020. The different general laboratory testing services agreements in the three months ended March 31, 2021 had an effect of gross margin as compared to the three months ended March 31, 2020. We estimate that our gross margin from general laboratory testing services segment will remain at its current quarterly level.
Our gross profit from immunology and hematology testing services for the three months ended March 31, 2021 was $212,359, with a gross margin of 70.4%. We did not generate any gross profit from immunology and hematology testing services in the three months ended March 31, 2020. We estimate that our gross margin from immunology and hematology testing services segment will remain at its current quarterly level.
80
Research and development expenses
Research and development expenses for the three months ended March 31, 2021 was $565,331, as compared to $410,893 for the three months ended March 31, 2020, an increase of $154,438, or 37.6%, which was mainly due to the increase in research and development projects. We expect that our research and development expenses will continue to increase in the near future.
General and administrative expenses
For the three months ended March 31, 2021 and 2020, general and administrative expenses consisted of the following:
| Three
Months Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| Depreciation | $ | 185,874 | $ | 144,961 | ||||
| Compensation and related benefits | 86,204 | 108,466 | ||||||
| Rent and related utilities | 63,285 | 74,701 | ||||||
| Professional fees | 24,041 | 1,145 | ||||||
| Other general and administrative | 46,784 | 14,979 | ||||||
| $ | 406,188 | $ | 344,252 | |||||
| ● | For the three months ended March 31, 2021, depreciation on property and equipment increased by $40,913, or 28.2%, as compared to the three months ended March 31, 2020, which was primarily attributable to an increase in depreciation on leasehold improvements since we made additional improvement to our leased office and commenced the amortization in the first quarter of 2021. We expect that our depreciation will remain in its current quarterly level in the near future. |
| ● | For the three months ended March 31, 2021, compensation and related benefits decreased by $22,262, or 20.5%, as compared to the three months ended March 31, 2020. The decrease was primarily attributable to a decrease in employees’ benefit. We expect that our compensation and related benefits will remain in its current quarterly level with minimal increase in the near future. |
| ● | For the three months ended March 31, 2021, rent and related utilities expenses decreased by $11,416, or 15.3%, as compared to the three months ended March 31, 2020. The decrease was primarily attributable to a decrease in Beijing office rent since we moved out. |
| ● | Professional fees primarily consisted of consulting fees and other fees. For the three months ended March 31, 2021, professional fees increased by $22,896, or 1,999.7%, as compared to the three months ended March 31, 2020. The increase was primarily attributable to an increase in use of professional services providers. We expect that our professional fees will remain in its current quarterly level with minimal increase in the near future. |
| ● | Other general and administrative expenses mainly consisted of office supplies, bank service charge, and other miscellaneous items. For the three months ended March 31, 2021, other general and administrative expenses increased by $31,805, or 212.3%, as compared to the three months ended March 31, 2020, resulting from business expansion. |
81
Selling and marketing expenses
For the three months ended March 31, 2021, selling and marketing expenses amounted to $52,707, as compared to $8,584 for the three months ended March 31, 2020, an increase of $44,123, or 514.0%. This increase was primarily attributable to the increase of our revenues. We expect our selling and marketing expenses will continue to increase since we anticipate that our revenues will continue to increase in the near future.
For the three months ended March 31, 2021, selling and marketing expenses as a percentage of total revenues decreased to 4.2% from 20.0% for the three months ended March 31, 2020. The decrease was primarily attributable to the increase in our revenues, as a portion of our selling and marketing expense are fixed and do not increase along with the increase in our revenue.
Grant income
For the three months ended March 31, 2021 and 2020, grant income amounted to $123,467 and $526,703, respectively.
Loss from operations
As a result of the foregoing, for the three months ended March 31, 2021, loss from operations amounted to $90,663, as compared to $208,256 for the three months ended March 31, 2020, a decrease of $117,593, or 56.5%.
Other (expense) income
Other (expense) income mainly includes interest expense and other miscellaneous income.
Other expense, net, totaled $16,104 for the three months ended March 31, 2021, as compared to $416 for the three months ended March 31, 2020, an increase of $15,688, or 3,771.2%, which was primarily attributable to an increase in interest expense of approximately $14,000 and a decrease in miscellaneous income of approximately $1,000.
Income taxes
Our income taxes expense is mainly attributable to our VIE in China, SenlangBio and its subsidiary, which were incorporated in the PRC and are subject to PRC Enterprise Income Tax (“EIT”) on the taxable income in accordance with the relevant PRC income tax laws. On March 16, 2007, the National People’s Congress enacted a new enterprise income tax law, which took effect on January 1, 2008, and was amended on December 29, 2018. The law applies a uniform 25% enterprise income tax rate to both foreign invested enterprises and domestic enterprises.
Income taxes expense was $28,513 for the three months ended March 31, 2021, resulting from taxable income generated by SenlangBio’s subsidiary.
We did not have any income taxes expense for the three months ended March 31, 2020 since we incurred losses in the first quarter of 2020.
Net loss
As a result of the factors described above, our net loss was $135,280 for the three months ended March 31, 2021, as compared to $208,672 for the three months ended March 31, 2020, a decrease of $73,392, or 35.2%.
Foreign currency translation adjustment
The reporting currency of the Company is the U.S. dollar. The functional currency of the parent company, Sen Lang, and Senlang HK, is the U.S. dollar and the functional currency of the PRC Subsidiary, SenlangBio, and SenlangBio Clinical Laboratory is the Chinese Renminbi (“RMB”). The financial statements of subsidiary, VIE and VIE’s subsidiary whose functional currency is the RMB are translated to U.S. dollars using period end rates of exchange for assets and liabilities, average rate of exchange for revenues, costs, and expenses and cash flows, and at historical exchange rates for equity. Net gains and losses resulting from foreign exchange translation is recorded as other comprehensive income (loss). Net gains and losses resulting from foreign exchange transactions are included in the results of operations. As a result of foreign currency translations, which are a non-cash adjustment, we reported a foreign currency translation loss of $577 and $47,085 as part of our other comprehensive loss for the three months ended March 31, 2021 and 2020, respectively. This non-cash loss had the effect of increasing our reported comprehensive loss.
82
Comprehensive Loss
As a result of our foreign currency translation adjustment, we had comprehensive loss of $135,857 and $255,757 for the three months ended March 31, 2021 and 2020, respectively.
Comparison of Results of Operations for the Years Ended December 31, 2020 and 2019
Revenues
For the year ended December 31, 2020, we had general laboratory testing services revenue of $649,932, as compared to $77,594 for the year ended December 31, 2019, an increase of $572,338, or 737.6%, resulting from our business expansion. We expect that our revenue from general laboratory testing services will continue to increase in the near future.
For the year ended December 31, 2020, we had immunology and hematology testing services revenue of $440,183, as compared to $15,736 for the year ended December 31, 2019, an increase of $424,447, or 2,697.3%, resulting from business expansion. We expect that our revenue from immunology and hematology testing services will continue to increase in the near future.
Costs of revenues
Costs of general laboratory testing services include the cost of inventory, labor and related benefits, depreciation, and other overhead costs.
For the year ended December 31, 2020, costs of general laboratory testing services amounted to $343,794, as compared to $35,972 for the year ended December 31, 2019, an increase of $307,822, or 855.7%. The increase was mainly due to the increase in general laboratory testing services revenue.
Costs of immunology and hematology testing services include the cost of inventory, labor and related benefits, depreciation, and other overhead costs.
For the year ended December 31, 2020, costs of immunology and hematology testing services amounted to $197,444, as compared to $10,269 for the year ended December 31, 2019, an increase of $187,175, or 1,822.7%. The increase was mainly due to the increase in immunology and hematology testing services revenue.
Gross profit
Gross profit from general laboratory testing services for the year ended December 31, 2020 was $306,138, as compared to $41,622 for the year ended December 31, 2019, a change of $264,516, or 635.5%. Gross margin decreased to 47.1% for the year ended December 31, 2020 from gross margin of 53.6% for the year ended December 31, 2019. The decrease was primarily attributable to the increase in the allocation of fixed costs, mainly consisting of depreciation, to cost of general laboratory testing services revenue, resulting from newly purchased property and equipment in year 2020.
Our gross profit from immunology and hematology testing services for the year ended December 31, 2020 was $242,739, as compared to $5,467 for the year ended December 31, 2019, a change of $237,272, or 4,340.1%. Gross margin increased to 55.1% for the year ended December 31, 2020 from gross margin of 34.7% for the year ended December 31, 2019. The significant increase in gross margin for the year ended December 31, 2020 as compared to the year ended December 31, 2019 were primarily attributable to the increased scale of operations resulting from higher revenue, resulting in fixed costs representing a much smaller proportion of revenue generated.
83
Research and development expenses
Research and development expenses for the year ended December 31, 2020 was $2,813,250, as compared to $2,624,879 for the year ended December 31, 2019, an increase of $188,371, or 7.2%, which was mainly due to the increase in research and development projects. We expect that our research and development expenses will continue to increase in the near future.
Selling and marketing expenses
For the year ended December 31, 2020, selling and marketing expenses amounted to $163,145, and selling and marketing expenses as a percentage of total revenues was 15.0%. There were no selling and marketing expenses in the year ended December 31, 2019 since we did not incur any selling and marketing activities.
General and administrative expenses
For the year ended December 31, 2020 and 2019, general and administrative expenses consisted of the following:
| Years Ended December 31, | ||||||||
| 2021 | 2020 | |||||||
| Compensation and related benefits | $ | 292,439 | $ | 504,488 | ||||
| Depreciation | 284,712 | 201,723 | ||||||
| Rent and related utilities | 143,537 | 145,251 | ||||||
| Professional fees | 82,591 | 30,909 | ||||||
| Office supplies | 35,490 | 72,752 | ||||||
| Other general and administrative | 86,669 | 65,702 | ||||||
| $ | 925,438 | $ | 1,020,825 | |||||
| ● | For the year ended December 31, 2020, compensation and related benefits decreased by $212,049, or 42.0%, as compared to the year ended December 31, 2019. The decrease was primarily attributable to a decrease in our administrative staff, reflecting our efforts at stricter controls on corporate expenditure. |
| ● | For the year ended December 31, 2020, depreciation on property and equipment increased by $82,989, or 41.1%, as compared to the year ended December 31, 2019, which was primarily attributable to an increase in depreciation on leasehold improvements since we made additional improvement to our leased office in 2020. |
| ● | For the year ended December 31, 2020, rent and related utilities expenses decreased by $1,714, or 1.2%, as compared to the year ended December 31, 2019. |
| ● | Professional fees primarily consisted of consulting fees and other fees. For the year ended December 31, 2020, professional fees increased by $51,682, or 167.2%, as compared to the year ended December 31, 2019. The increase was primarily attributable to an increase in use of professional services providers. |
| ● | For the year ended December 31, 2020, office supplies decreased by $37,262, or 51.2%, as compared to the year ended December 31, 2019, reflecting our efforts at stricter controls on corporate expenditure. |
| ● | Other general and administrative expenses mainly consisted of bank service charge and other miscellaneous items. For the year ended December 31, 2020, other general and administrative expenses increased by $20,967, or 31.9%, as compared to the year ended December 31, 2019. The increase was mainly due to our business expansion. |
84
Grant income
For the year ended December 31, 2020 and 2019, grant income amounted to $929,505 and $612,769, respectively.
Loss from operations
As a result of the foregoing, for the year ended December 31, 2020, loss from operations amounted to $2,423,451, as compared to $2,985,846 for the year ended December 31, 2019, a decrease of $562,395, or 18.8%.
Other (expense) income
Other (expense) income mainly includes interest expense and other miscellaneous income.
Other expense, net, totaled $18,912 for the year ended December 31, 2020, as compared to other income, net, of $6,942 for the year ended December 31, 2019, an increase of $25,854, or 372.4%, which was primarily attributable to an increase in interest expense of approximately $24,000 and a decrease in miscellaneous income of approximately $2,000.
Income taxes
Our income taxes expense is mainly attributable to our VIE in China, SenlangBio and its subsidiary, which were incorporated in the PRC and are subject to PRC Enterprise Income Tax (“EIT”) on the taxable income in accordance with the relevant PRC income tax laws. On March 16, 2007, the National People’s Congress enacted a new enterprise income tax law, which took effect on January 1, 2008, and was amended on December 29, 2018. The law applies a uniform 25% enterprise income tax rate to both foreign invested enterprises and domestic enterprises.
We did not have any income taxes expense for the year ended December 31, 2020 and 2019 since we incurred losses in these periods.
Net loss
As a result of the factors described above, our net loss was $2,442,363 for the year ended December 31, 2020, as compared to $2,978,904 for the year ended December 31, 2019, a decrease of $536,541 or 18.0%.
Foreign currency translation adjustment
The reporting currency of the Company is the U.S. dollar. The functional currency of the parent company, Sen Lang, and Senlang HK, is the U.S. dollar and the functional currency of the PRC Subsidiary, SenlangBio, and SenlangBio Clinical Laboratory is the Chinese Renminbi (“RMB”). The financial statements of subsidiary, VIE and VIE’s subsidiary whose functional currency is the RMB are translated to U.S. dollars using period end rates of exchange for assets and liabilities, average rate of exchange for revenues, costs, and expenses and cash flows, and at historical exchange rates for equity. Net gains and losses resulting from foreign exchange translation are recorded as other comprehensive income (loss). Net gains and losses resulting from foreign exchange transactions are included in the results of operations. As a result of foreign currency translations, which are a non-cash adjustment, we reported a foreign currency translation gain of $58,807 and a foreign currency translation loss of $50,204 as other comprehensive income (loss) for the year ended December 31, 2020 and 2019, respectively. This non-cash gain/loss had the effect of decreasing/increasing our reported comprehensive loss.
Comprehensive Loss
As a result of our foreign currency translation adjustment, we had comprehensive loss of $2,383,556 and $3,029,108 for the year ended December 31, 2020 and 2019, respectively.
Liquidity and Capital Resources
The Company has a limited operating history and its continued growth is dependent upon the providing general laboratory testing and immunology and hematology testing services for patients and other customers in China; hence generating revenues, and obtaining additional financing to fund future obligations and pay liabilities arising from normal business operations. In addition, the current cash balance cannot be projected to cover the operating expenses for the next twelve months from the release date of this report. These matters raise substantial doubt about the Company’s ability to continue as a going concern. The ability of the Company to continue as a going concern is dependent on the Company’s ability to raise additional capital, implement its business plan, and generate significant revenues. There are no assurances that the Company will be successful in its efforts to generate significant revenues, maintain sufficient cash balance or report profitable operations or to continue as a going concern. The Company plans on raising capital through the sale of equity to implement its business plan. However, there is no assurance these plans will be realized and that any additional financings will be available to the Company on satisfactory terms and conditions, if any.
85
The occurrence of an uncontrollable event such as the COVID-19 pandemic is likely to negatively affect the Company’s operations. Given the dynamic nature of these circumstances, the related financial effect cannot be reasonably estimated at this time but is expected to adversely impact our business for the rest of year 2021.
Liquidity is the ability of a company to generate funds to support its current and future operations, satisfy its obligations and otherwise operate on an ongoing basis. At March 31, 2021 and December 31, 2020, we had cash balance of approximately $189,000 and $19,000, respectively. These funds are kept in financial institutions in the PRC.
Under applicable PRC regulations, foreign invested enterprises, or FIEs, in China may pay dividends only out of their accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. In addition, a foreign invested enterprise in China is required to set aside at least 10% of its after-tax profit based on PRC accounting standards each year to its general reserves until the cumulative amount of such reserves reach 50% of its registered capital. These reserves are not distributable as cash dividends.
In addition, a portion of our businesses and assets are denominated in RMB, which is not freely convertible into foreign currencies. All foreign exchange transactions take place either through the People’s Bank of China or other banks authorized to buy and sell foreign currencies at the exchange rates quoted by the People’s Bank of China. Approval of foreign currency payments by the People’s Bank of China or other regulatory institutions requires submitting a payment application form together with suppliers’ invoices, shipping documents and signed contracts. These currency exchange control procedures imposed by the PRC government authorities may restrict the ability of our PRC subsidiary to transfer its net assets to the Parent Company through loans, advances or cash dividends.
The current PRC Enterprise Income Tax (“EIT”) Law and its implementing rules generally provide that a 10% withholding tax applies to China-sourced income derived by non-resident enterprises for PRC enterprise income tax purposes unless the jurisdiction of incorporation of such enterprises’ shareholder has a tax treaty with China that provides for a different withholding arrangement.
The following table sets forth a summary of changes in our working capital from December 31, 2020 to March 31, 2021:
| March 31, | December 31, | Changes in | ||||||||||||||
| 2021 | 2020 | Amount | Percentage | |||||||||||||
| Working capital deficit: | ||||||||||||||||
| Total current assets | $ | 1,336,080 | $ | 546,335 | $ | 789,745 | (144.6 | )% | ||||||||
| Total current liabilities | 3,110,083 | 2,437,564 | 672,519 | (27.6 | )% | |||||||||||
| Working capital deficit | $ | (1,774,003 | ) | $ | (1,891,229 | ) | $ | 117,226 | (6.2 | )% | ||||||
Our working capital deficit decreased by $117,226 to $1,774,003 at March 31, 2021 from $1,891,229 at December 31, 2020. The decrease in working capital deficit was primarily attributable to an increase in cash of approximately $170,000, an increase in accounts receivable of approximately $591,000, mainly due to the increase in revenues, a decrease in accrued leasehold improvements liabilities of approximately $54,000, and a decrease in deferred grant income of approximately $77,000, offset by an increase in notes payable of approximately $455,000, an increase in accounts payable of approximately $254,000, and an increase in deferred revenue of approximately $79,000.
86
Because the exchange rate conversion is different for the condensed consolidated balance sheets and the condensed consolidated statements of cash flows, the changes in assets and liabilities reflected on the condensed consolidated statements of cash flows are not necessarily identical with the comparable changes reflected on the condensed consolidated balance sheets.
Cash Flows for the Three Months Ended March 31, 2021 Compared to the Three Months Ended March 31, 2020
The following summarizes the key components of our cash flows for the three months ended March 31, 2021 and 2020:
| Three Months Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| Net cash (used in) provided by operating activities | $ | (233,267 | ) | $ | 13,809 | |||
| Net cash used in investing activities | (57,581 | ) | (18,665 | ) | ||||
| Net cash provided by financing activities | 462,656 | 214,829 | ||||||
| Effect of exchange rate on cash | (1,856 | ) | (5,744 | ) | ||||
| Net increase in cash | $ | 169,952 | $ | 204,229 | ||||
Net cash flow used in operating activities for the three months ended March 31, 2021 was $233,267, which primarily reflected our consolidated net loss of approximately $135,000, and the changes in operating assets and liabilities, primarily consisting of an increase in accounts receivable of approximately $598,000, mainly due to the increase in revenues, an increase in prepaid expenses and other current assets of approximately $40,000, a decrease in deferred grant income of approximately $123,000, and a decrease in operating lease obligation of approximately $57,000, offset by an increase in accounts payable of approximately $258,000, an increase in deferred revenue of approximately $80,000, and the non-cash items adjustment primarily consisting of depreciation of approximately $297,000, and amortization of right-of-use assets of approximately $53,000.
Net cash flow provided by operating activities for the three months ended March 31, 2020 was $13,809, which primarily reflected the add-back of non-cash items mainly consisting of depreciation of approximately $252,000, and amortization of right-of-use assets of approximately $45,000, and the changes in operating assets and liabilities mainly consisting of a decrease in prepaid expenses and other current assets of approximately $33,000, an increase in accounts payable of approximately $35,000, offset by a decrease in deferred grant income of approximately $43,000, a decrease in operating lease obligation of approximately $46,000, and our consolidated net loss of approximately $209,000.
We expect our cash used in operating activities to increase due to the following:
| ● | the development and commercialization of new services or products; |
| ● | an increase in professional staff; and |
| ● | an increase in sales promotions for existing and/or new brands as we expand within existing markets or enter new markets. |
Net cash flow used in investing activities reflects the purchase of property and equipment of $57,581 and $18,665 for the three months ended March 31, 2021 and 2020, respectively.
Net cash flow provided by financing activities was $462,656 for the three months ended March 31, 2021 as compared to $214,829 for the three months ended March 31, 2020. During the three months ended March 31, 2021, we received proceeds from third party borrowings of approximately $463,000. During the three months ended March 31, 2020, we received proceeds from related party borrowings of approximately $215,000.
87
Cash Flows for the Year Ended December 31, 2020 Compared to the Year Ended December 31, 2019
The following summarizes the key components of our cash flows for the years ended December 31, 2020 and 2019:
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Net cash used in operating activities | $ | (1,121,944 | ) | $ | (2,192,353 | ) | ||
| Net cash used in investing activities | (128,109 | ) | (881,688 | ) | ||||
| Net cash provided by financing activities | 1,101,465 | - | ||||||
| Effect of exchange rate on cash | 2,529 | (16,066 | ) | |||||
| Net decrease in cash | $ | (146,059 | ) | $ | (3,090,107 | ) | ||
Net cash flow used in operating activities for the year ended December 31, 2020 was $1,121,944, which primarily reflected our consolidated net loss of approximately $2,442,000, and the changes in operating assets and liabilities, primarily consisting of an increase in accounts receivable of approximately $41,000, a decrease in deferred grant income of approximately $93,000, and a decrease in operating lease obligation of approximately $190,000, offset by a decrease in prepaid expenses and other current assets of approximately $39,000, an increase in accounts payable of approximately $214,000, an increase in deferred revenue of approximately $81,000, and the non-cash items adjustment primarily consisting of depreciation of approximately $1,147,000, and amortization of right-of-use assets of approximately $189,000.
Net cash flow used in operating activities for the year ended December 31, 2019 was $2,192,353, which primarily reflected our consolidated net loss of approximately $2,979,000, and the changes in operating assets and liabilities, primarily consisting of an increase in recoverable VAT of approximately $94,000, an increase in inventory of approximately $93,000, a decrease in deferred grant income of approximately $158,000, and a decrease in operating lease obligation of approximately $174,000, offset by a decrease in prepaid expenses and other current assets of approximately $124,000, an increase in accounts payable of approximately $35,000, an increase in salary payable of approximately $50,000, an increase in accrued liabilities and other payables of approximately $35,000, and the non-cash items adjustment primarily consisting of depreciation of approximately $889,000, and amortization of right-of-use assets of approximately $169,000.
Net cash flow used in investing activities was $128,109 for the year ended December 31, 2020 as compared to $881,688 for the year ended December 31, 2019. During the year ended December 31, 2020, we made payment for purchase of property and equipment of approximately $128,000.
During the year ended December 31, 2019, we made payment for purchase of property and equipment of approximately $870,000, and made prepayment for property and equipment of approximately $12,000.
Net cash flow provided by financing activities was $1,101,465 for the year ended December 31, 2020. During the year ended December 31, 2020, we received proceeds from third party borrowings of $870,000 and proceeds from related party borrowings of $609,000, offset by repayments made for related party borrowings of $377,000.
We did not have any financing activity during the year ended December 31, 2019.
88
Our capital requirements for the next twelve months primarily relate to working capital requirements, including purchase of inventory, salaries, reduction of accrued liabilities, repayment of third party and related party borrowings, and the development of business opportunities. These uses of cash will depend on numerous factors including our revenues and our ability to control costs. All funds received have been expended in the furtherance of growing the business. The following trends are reasonably likely to result in a material decrease in our liquidity over the near to long term:
| ● | an increase in working capital requirements to finance our current business, including ongoing research and development programs, as well as commercial strategies; |
| ● | the use of capital for the development of business opportunities; and |
| ● | addition of administrative personnel as the business grows. |
We estimate that based on current plans and assumptions, that our available cash will be insufficient to satisfy our cash requirements under our present operating expectations. Other than funds received from advances from our related party and/or third party, and cash resource generating from our operations, we presently have no other significant alternative source of working capital. We have used these funds to fund our operating expenses, pay our obligations and grow our company. We will need to raise significant additional capital to fund our operations and to provide working capital for our ongoing operations and obligations. Therefore, our future operation is dependent on our ability to secure additional financing. Financing transactions may include the issuance of equity or debt securities, obtaining credit facilities, or other financing mechanisms. The inability to obtain additional capital may restrict our ability to grow and may reduce our ability to continue to conduct business operations. If we are unable to obtain additional financing, we will be required to cease our operations. To date, we have not considered this alternative, nor do we view it as a likely occurrence.
Avalon and SenlangBio currently expect that SenlangBio’s cash reserves, based on cash balances at March 31, 2021, together with the proceeds from the Equity Financing, will fund SenlangBio’s operations for 18 months following the closing of the Acquisition, based on current plans and assumptions.
Contractual Obligations and Off-Balance Sheet Arrangements
Contractual Obligations
We have certain fixed contractual obligations and commitments that include future estimated payments. Changes in our business needs, cancellation provisions, and other factors may result in actual payments differing from the estimates. We cannot provide certainty regarding the timing and amounts of payments. We have presented below a summary of the most significant assumptions used in our determination of amounts presented in the tables, in order to assist in the review of this information within the context of our consolidated financial position, results of operations, and cash flows. The following tables summarize our contractual obligations as of March 31, 2021, and the effect these obligations are expected to have on our liquidity and cash flows in future periods.
| Payments Due by Period | ||||||||||||||||||||
| Contractual obligations: | Total | Less than 1 year | 1-3 years | 3-5 years | 5+ years | |||||||||||||||
| Operating lease commitment | $ | 215,680 | $ | 162,681 | $ | 52,999 | $ | - | $ | - | ||||||||||
| Borrowings from third party (principal) | 1,373,480 | 1,373,480 | - | - | - | |||||||||||||||
| Borrowings from related party (principal) | 244,174 | 244,174 | - | - | - | |||||||||||||||
| Accrued interest | 8,907 | 8,907 | - | - | - | |||||||||||||||
| Total | $ | 1,842,241 | $ | 1,789,242 | $ | 52,999 | $ | - | $ | - | ||||||||||
Off-balance Sheet Arrangements
The Company did not have during the periods presented, and it does not currently have, any off-balance sheet arrangements, as defined under SEC rules, such as relationships with unconsolidated entities or financial partnerships, which are often referred to as structured finance or special purpose entities, established for the purpose of facilitating financing transactions that are not required to be reflected on its consolidated balance sheets.
89
Quantitative and Qualitative Disclosures about Market Risk
Interest Rate Risk
None of the Company’s debt contain interest rate provisions that may be subject to fluctuations in market interest rates. As such, the Company does not have significant interest rate risk or has entered into any financial instruments to mitigate such risk.
We do not use financial instruments for trading purposes and are not parties to any leverage derivatives. We do not currently engage in hedging transactions.
Credit Risk
Credit risk is controlled by the application of credit approvals, limits and monitoring procedures. We manage credit risk through in-house research and analysis of the Chinese economy and the underlying obligors and transaction structures. We identify credit risk collectively based on industry, geography and customer type. In measuring the credit risk of our services to our customers, we mainly reflect the “probability of default” by the customer on its contractual obligations and consider the current financial position of the customer and the current and likely future exposures to the customer.
Liquidity Risk
We are also exposed to liquidity risk which is risk that we will be unable to provide sufficient capital resources and liquidity to meet our commitments and business needs. Liquidity risk is controlled by the application of financial position analysis and monitoring procedures. When necessary, we will turn to other financial institutions and related parties to obtain short-term funding to cover any liquidity shortage.
Foreign Currency Exchange Rate Risk
Our operations are in China. Thus, our revenues and operating results may be impacted by exchange rate fluctuations between RMB and U.S. dollars. For the three months ended March 31, 2021 and 2020, we had an unrealized foreign currency translation loss of approximately $600 and $47,100, respectively, because of changes in the exchange rate.
Inflation
The effect of inflation on our revenue and operating results was not significant.
90
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Other than compensation arrangements for executive officers and directors, described below is each transaction and series of similar transactions, since the beginning of fiscal year 2019, to which Sen Lang and Avalon, respectively, was a party or will be a party, in which:
| ● | the amounts involved exceeded or will exceed the lesser of $120,000 or one percent of the average of the company’s total assets at year-end for the last two completed fiscal years; and |
| ● | any of the company’s directors, nominees for director, executive officers or holders of more than 5% of the company’s common stock, or any member of the immediate family of the foregoing persons, had or will have a direct or indirect material interest. |
Compensation arrangements for Avalon’s named executive officers and directors are described in the section entitled “Avalon’s Corporate Governance and Executive Compensation.”
Sen Lang
From time to time, Sen Lang acquires loans from Hebei Senlang Taihe Biological Technology Co. Ltd. (“Taihe”) to fund its operations. These loans are due within one year and are unsecured and uncollateralized. The annual interest rate for these loans is 4.35%. SenlangBio’s largest shareholder is the former executive director, and currently holds 80% of the equity interest, of Taihe.
As of March 31, 2021 and December 31, 2020, the outstanding principal amounted to $244,174 and $245,000, respectively, and were recorded as “Notes payable – related party” on the condensed consolidated balance sheets included herein.
For the three months ended March 31, 2021 and 2020, interest expense related to related party borrowings amounted to $2,683 and $1,860, respectively. For the year ended December 31, 2020, interest expense related to related party borrowings amounted to $11,169. As of both March 31, 2021 and December 31, 2020, the related interest for related party borrowings was paid in full.
In May 2021, Sen Lang borrowed a short-term loan from Taihe in the principal amount of $763,044. The loan bears interest rate at 4.35% per annum and is due on October 31, 2021.
On April 10, 2020, in the ordinary course of business, SenlangBio entered into a scientific research project cooperation agreement with Beijing Lu Daopei Hospital Co., Ltd., under which Beijing Lu Daopei Hospital Co., Ltd. conducts scientific research for the interest of SenlangBio on the cytoplasmic CD79a antibody gated multicolor flow cytometry monitoring CD19-CAR-T bridging allogeneic transplantation for the treatment of refractory and relapsed acute B lymphocytic leukemia. SenlangBio provides research funds in the amount of RMB 2 million to Beijing Lu Daopei Hospital Co., Ltd. Beijing Lu Daopei Hospital Co., Ltd. is a wholly-owned subsidiary of an entity whose chairman is Wenzhao Lu, the Chairman and largest shareholder of Avalon.
91
Avalon
Medical Related Consulting Services Revenue from Related Parties and Accounts Receivable – Related Party
During the years ended December 31, 2020 and 2019, medical related consulting services revenue from related parties was as follows:
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Medical related consulting services provided to: | ||||||||
| Beijing Daopei * | $ | - | $ | 54,909 | ||||
| Shanghai Daopei * | 170,908 | 13,926 | ||||||
| Hebei Daopei * | - | 286,709 | ||||||
| $ | 170,908 | $ | 355,544 | |||||
| * | Beijing Daopei, Shanghai Daopei, and Hebei Daopei are subsidiaries of an entity whose chairman is Wenzhao Lu, the largest shareholder of Avalon. |
As of December 31, 2020, accounts receivable – related party was $0. Accounts receivable – related party at December 31, 2019 amounted to $215,418 and no allowance for doubtful accounts is deemed to be required on accounts receivable – related party at December 31, 2019.
Accrued Liabilities and Other Payables – Related Parties
Avalon acquired Beijing Genexosome for a cash payment of $450,000. As of December 31, 2020 and 2019, the unpaid acquisition consideration of $100,000, was payable to Dr. Yu Zhou, former director and former co-chief executive officer and 40% owner of Genexosome, and has been included in accrued liabilities and other payables – related parties on the accompanying consolidated balance sheets.
As of December 31, 2020 and 2019, the accrued and unpaid interest related to borrowings from Wenzhao Lu, Avalon’s largest shareholder and chairman of the Board of Directors, amounted to $167,956 and $49,194, respectively, and have been included in accrued liabilities and other payables – related parties on the accompanying consolidated balance sheets.
Borrowings from Related Party
Promissory Note
On March 18, 2019, Avalon issued Wenzhao Lu, Avalon’s largest shareholder and Chairman of the Board of Directors, a Promissory Note in the principal amount of $1,000,000 (“Promissory Note”) in consideration of cash in the amount of $1,000,000. The Promissory Note accrues interest at the rate of 5% per annum and matures March 19, 2022. Avalon repaid principal of $410,000 and $200,000 in the third quarter of 2019 and second quarter of 2020, respectively. As of December 31, 2020 and 2019, the outstanding principal balance was $390,000 and $590,000, respectively.
92
Line of Credit
On August 29, 2019, Avalon entered into a Line of Credit Agreement (the “Line of Credit Agreement”) providing Avalon with a $20 million line of credit (the “Line of Credit”) from Wenzhao Lu (the “Lender”), the largest shareholder and Chairman of the Board of Directors of Avalon. The Line of Credit allows Avalon to request loans thereunder and to use the proceeds of such loans for working capital and operating expense purposes until the facility matures on December 31, 2024. The loans are unsecured and are not convertible into equity of Avalon. Loans drawn under the Line of Credit bears interest at an annual rate of 5% and each individual loan will be payable three years from the date of issuance. Avalon has a right to draw down on the line of credit and not at the discretion of the related party Lender. Avalon may, at its option, prepay any borrowings under the Line of Credit, in whole or in part at any time prior to maturity, without premium or penalty. The Line of Credit Agreement includes customary events of default. If any such event of default occurs, the Lender may declare all outstanding loans under the Line of Credit to be due and payable immediately. As of December 31, 2020 and 2019, $3,200,000 and $2,600,000 was outstanding under the Line of Credit, respectively.
For the years ended December 31, 2020 and 2019, the interest expense related to above borrowings amounted to $168,762 and $49,194, respectively, and has been included in interest expense – related party on the accompanying consolidated statements of operations and comprehensive loss.
As of December 31, 2020 and 2019, the related accrued and unpaid interest for above borrowings was $167,956 and $49,194, respectively, and has been included in accrued liabilities and other payables – related parties on the accompanying consolidated balance sheets.
Common Shares Sold to Related Party
On April 1, 2020, Avalon sold 645,161 shares of its common stock to WLM Limited (“WLM”), an entity owned by Wenzhao Lu, Chairman of the Board of Directors of Avalon, at a price per share of $1.55 for an aggregate purchase price of $1,000,000.
Office Space from Related Party
Beijing Genexosome uses office space of a related party, free of rent, which is considered immaterial.
93
Avalon GloboCare Corp. is a clinical-stage, vertically integrated, leading CellTech bio-developer dedicated to advancing and empowering innovative, transformative immune effector cell therapy, exosome technology, as well as COVID-19 related diagnostics and therapeutics. The Company also provides strategic advisory and outsourcing services to facilitate and enhance its clients' growth and development, as well as competitiveness in healthcare and CellTech industry markets. Through its subsidiary structure with unique integration of verticals from innovative R&D to automated bioproduction and accelerated clinical development, the Company is establishing a leading role in the fields of cellular immunotherapy (including CAR-T/NK), exosome technology (ACTEX™), and regenerative therapeutics.
The principal executive offices of Avalon are located at 4400 Route 9 South, Suite 3100, Freehold, New Jersey 07728, and its telephone number is (732) 780-4400.
For additional information regarding Avalon, please refer to its Annual Report on Form 10-K for the year ended December 31, 2020, which Annual Report is being sent to stockholders together with this proxy statement.
94
AVALON’S CORPORATE GOVERNANCE AND EXECUTIVE COMPENSATION
The Board of Directors oversees Avalon’s business and affairs and monitors the performance of management. In accordance with corporate governance principles, the Board does not involve itself in day-to-day operations. The directors keep themselves informed through discussions with the Chief Executive Officer and other key executives, visits to the Company’s facilities, by reading the reports and other materials that Avalon sends them and by participating in Board and committee meetings. Each director’s term will continue until the election and qualification of his or her successor, or his or her earlier death, resignation or removal. Biographical information about the Company’s directors is provided in “Proposal No. 1 — The Director Election Proposal” in this proxy statement. Except as set forth in this proxy statement, none of the directors held directorships in other reporting companies or registered investment companies at any time during the past five years. The Board currently consists of nine persons, all of whom have been nominated by the Company to stand for re-election.
| Name | Age | Position | ||
| Wenzhao “Daniel” Lu | 63 | Chairman of the Board of Directors | ||
| David Jin, MD, PhD | 53 | Chief Executive Officer, President and Director | ||
| Meng Li | 43 | Chief Operating Officer, Secretary and Director | ||
| Steven A. Sanders | 75 | Director | ||
| Yancen Lu | 46 | Director | ||
| Wilbert J. Tauzin II | 76 | Director | ||
| William B. Stilley, III | 53 | Director | ||
| Tevi Troy | 53 | Director | ||
| Yue “Charles” Li | 47 | Director |
Board Composition
The Company’s business and affairs are organized under the direction of its board of directors, which currently consists of nine members. The primary responsibility of the board of directors is to provide oversight, strategic guidance, counseling, and direction to the Company’s management team. The board of directors meets on a regular basis and additionally as required.
A majority of the authorized number of directors constitutes a quorum of the Board of Directors for the transaction of business. The directors must be present at the meeting to constitute a quorum. However, any action required or permitted to be taken by the Board of Directors may be taken without a meeting if all members of the Board of Directors individually or collectively consent in writing to the action.
Director Independence
The board of directors currently consists of nine (9) members. The board of directors has determined that Yancen Lu, William B. Stilley, III, Steven A. Sanders, Tevi Troy and Yue “Charles” Li, qualify as independent directors in accordance with the Nasdaq Capital Market (“Nasdaq”) listing requirements. Mr. Wenzhao Lu, Dr. Jin, Meng Li and Wilbert Tauzin II are not considered independent. Nasdaq’s independence definition includes a series of objective tests, such as that the director is not, and has not been for at least three (3) years, one of the Company’s employees and that neither the director nor any of his or her family members has engaged in various types of business dealings with the Company. In addition, as required by Nasdaq rules, the board of directors has made a subjective determination as to each independent director that no relationships exist that, in the opinion of the board of directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. In making these determinations, the board of directors reviewed and discussed information provided by the directors and the Company with regard to each director’s business and personal activities and relationships as they may relate to the Company and its management.
95
As required under Nasdaq rules and regulations, the independent directors meet in regularly scheduled executive sessions at which only independent directors are present.
Family Relationships
There are no family relationships among the directors or executive officers.
Board Leadership Structure and Role in Risk Oversight
Avalon’s Board of Directors, or the Board, is primarily responsible for overseeing the Company’s risk management processes on behalf of the company. The Board receives and reviews periodic reports from management, auditors, legal counsel, and others, as considered appropriate regarding the Company’s assessment of risks. In addition, the Board focuses on the most significant risks facing the Company and the Company’s general risk management strategy, and also ensures that risks undertaken by the Company are consistent with the board’s appetite for risk. While the Board oversees the Company’s risk management, management is responsible for day-to-day risk management processes. Avalon believes this division of responsibilities is the most effective approach for addressing the risks facing the Company and that the board leadership structure supports this approach.
Involvement in Certain Legal Proceedings
To the Company’s knowledge, the Company’s directors and executive officers have not been involved in any of the following events during the past ten years:
| ● | any bankruptcy petition filed by or against such person or any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; |
| ● | any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); |
| ● | being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from or otherwise limiting his involvement in any type of business, securities or banking activities or to be associated with any person practicing in banking or securities activities; |
| ● | being found by a court of competent jurisdiction in a civil action, the SEC or the Commodity Futures Trading Commission to have violated a Federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; |
| ● | being subject of, or a party to, any Federal or state judicial or administrative order, judgment decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of any Federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
| ● | being subject of or party to any sanction or order, not subsequently reversed, suspended, or vacated, of any self-regulatory organization, any registered entity or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
96
Board Committees
Establishment of Board Committees and Adoption of Charters
In November 2018, the Company established a Nominating and Corporate Governance Committee, a Compensation Committee and an Audit Committee (collectively, the “Committees”) and approved and adopted charters to govern each of the Committees.
In connection with the establishment of the Nominating and Corporate Governance Committee, Compensation Committee and Audit Committee, the Board of Directors of the Company appointed members to each such committee. Currently, all three committees are comprised of at least three (3) directors meeting the requirements set forth in each applicable charter. The membership of these three standing committees of the Board of Directors of the Company is as follows:
| Nominating
and Corporate Governance Committee | Compensation Committee | Audit Committee | ||
| Steven Sanders (Chairman) | Yancen Lu (Chairman) | William Stilley (Chairman) | ||
| Tevi Troy | Steven Sanders | Yancen Lu | ||
| William Stilley | Tevi Troy | Steve Sanders |
Nominating and Corporate Governance Committee
Avalon’s board of directors has determined that each of the members of the Nominating and Governance Committee (the “Governance Committee”) are “independent directors” as defined by Nasdaq. The Governance Committee generally responsible for recommending to the full board of directors policies, procedures, and practices designed to help ensure that the Company’s corporate governance policies, procedures, and practices continue to assist the board of directors and management in effectively and efficiently promoting the best interests of the Company’s stockholders. The Governance Committee is also responsible for selecting and recommending for approval by the Company’s board of directors and stockholders a slate of director nominees for election at each of the Company’s annual meetings of stockholders, and otherwise for determining the board committee members and chairmen, subject to board of directors ratification, as well as recommending to the board director nominees to fill vacancies or new positions on the board of directors or its committees that may occur or be created from time to time, all in accordance with the Company’s bylaws and applicable law. The Governance Committee’s principal functions include:
| ● | developing and maintaining corporate governance policy guidelines; | |
| ● | developing and maintaining codes of conduct and ethics; | |
| ● | overseeing the interpretation and enforcement of the Code of Conduct and Code of Ethics for Chief Executive Officer and Senior Financial and Accounting Officers; | |
| ● | evaluating the performance of the board of directors, its committees, and committee chairmen and directors; and | |
| ● | selecting and recommending a slate of director nominees for election at each of the Company’s annual meetings of the stockholders and recommending to the board director nominees to fill vacancies or new positions on the board of directors or its committees that may occur from time to time. |
During 2020, the Nominating and Corporate Governance Committee did not meet. The Governance Committee is governed by a written charter approved by the board of directors. A copy of the Governance Committee’s charter is posted on the Company’s website at www.avalon-globocare.com in the “Investors” section of the website. In identifying potential independent board of directors’ candidates with significant senior-level professional experience, the Governance Committee solicits candidates from the board of directors, senior management and others and may engage a search firm in the process. The Governance Committee reviews and narrows the list of candidates and interviews potential nominees. The final candidate is also introduced and interviewed by the board of directors and the lead director if one has been appointed. In general, in considering whether to recommend any particular candidate for inclusion in the board of directors’ slate of recommended director nominees, the Governance Committee will apply the criteria set forth in its corporate governance guidelines. These criteria include the candidate’s integrity, business acumen, commitment to understanding our business and industry, experience, conflicts of interest and the ability to act in the interests of our stockholders. Further, specific consideration is given to, among other things, diversity of background and experience that a candidate would bring to the board of directors. The Governance Committee does not assign specific weights to particular criteria and no particular criterion is a prerequisite for each prospective nominee. The Company believe that the backgrounds and qualifications of its directors, considered as a group, should provide a composite mix of experience, knowledge and abilities that will allow the board of directors to fulfill its responsibilities. Stockholders may recommend individuals to the Governance Committee for consideration as potential director candidates by submitting their names, together with appropriate biographical information and background materials to the Company’s Governance Committee. Assuming that appropriate biographical and background material has been provided on a timely basis, the Governance Committee will evaluate stockholder recommended candidates by following substantially the same process, and applying substantially the same criteria, as it follows for candidates submitted by others.
97
Audit Committee
The Company has a separately-designated standing Audit Committee established in accordance with Section 3(a)(58)(A) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). The board of directors has determined that the members are all “independent directors” as defined by the rules of Nasdaq applicable to members of an audit committee and Rule 10A-3(b)(i) under the Exchange Act. In addition, Mr. Stilley is an “audit committee financial expert” as defined in Item 407(d)(5) of Regulation S-K and demonstrates “financial sophistication” as defined by the rules of The NASDAQ Stock Market, Inc. The Audit Committee is appointed by the board of directors to assist the board of directors in monitoring (1) the integrity of our financial statements, (2) the Company’s compliance with legal and regulatory requirements, and (3) the independence and performance of the Company’s internal and external auditors. The Audit Committee’s principal functions include:
| ● | reviewing the Company’s annual audited financial statements with management and the Company’s independent auditors, including major issues regarding accounting and auditing principles and practices and financial reporting that could significantly affect the Company’s financial statements; |
| ● | reviewing the Company’s quarterly financial statements with management and the Company’s independent auditor prior to the filing of the Company’s Quarterly Reports on Form 10-Q, including the results of the independent auditors’ reviews of the quarterly financial statements; |
| ● | recommending to the board of directors the appointment of, and continued evaluation of the performance of, the Company’s independent auditor; |
| ● | approving the fees to be paid to the Company’s independent auditor for audit services and approving the retention of the Company’s independent auditor for non-audit services and all fees for such services; |
| ● | reviewing periodic reports from the Company’s independent auditor regarding the Company’s auditor’s independence, including discussion of such reports with the auditor; |
| ● | reviewing the adequacy of the Company’s overall control environment, including internal financial controls and disclosure controls and procedures; and |
| ● | reviewing with the Company’s management and legal counsel legal matters that may have a material impact on the Company’s financial statements or the Company’s compliance policies and any material reports or inquiries received from regulators or governmental agencies. |
During 2020, the audit committee met four times. A copy of the Audit Committee’s charter is posted on the Company’s website at www.avalon-globocare.com in the “Investors” section of the website.
Meetings may be held from time to time to consider matters for which approval of the Company’s Board of Directors is desirable or is required by law.
98
Compensation Committee
Avalon’s compensation committee consists of Yancen Lu, Steven Sanders and Tevi Troy. The Company’s board of directors has determined that each of the members are an “independent director” as defined by the Nasdaq rules applicable to members of a compensation committee. The Compensation Committee is responsible for establishing the compensation of the Company’s senior management, including salaries, bonuses, termination arrangements, and other executive officer benefits as well as director compensation. The Compensation Committee also administers our equity incentive plans. During the year ended December 31, 2020, the Compensation Committee met one time. The Compensation Committee is governed by a written charter approved by the board of directors. A copy of the Compensation Committee’s charter is posted on the Company’s website at www.avalon-globocare.com in the “Investors” section of the website. The Compensation Committee works with the Chairman of the Board and Chief Executive Officer and reviews and approves compensation decisions regarding senior management including compensation levels and equity incentive awards. The Compensation Committee also approves employment and compensation agreements with the Company’s key personnel and directors. The Compensation Committee has the power and authority to conduct or authorize studies, retain independent consultants, accountants or others, and obtain unrestricted access to management, the Company’s internal auditors, human resources and accounting employees and all information relevant to its responsibilities.
The responsibilities of the Compensation Committee, as stated in its charter, include the following:
| ● | review and approve the Company’s compensation guidelines and structure; |
| ● | review and approve on an annual basis the corporate goals and objectives with respect to compensation for the Chief Executive Officer; |
| ● | review and approve on an annual basis the evaluation process and compensation structure for the Company’s other officers, including salary, bonus, incentive and equity compensation; and |
| ● | periodically review and make recommendations to the Board of Directors regarding the compensation of non-management directors. |
The Compensation Committee is responsible for developing the executive compensation philosophy and reviewing and recommending to the Board of Directors for approval all compensation policies and compensation programs for the executive team.
Compensation Committee Interlocks and Insider Participation
None of the Company’s executive officers currently serves, or in the past year has served, as a member of the board of directors or compensation committee of any entity that has one or more executive officers on the Company’s board of directors or compensation committee.
Code of Ethics
The Company has a code of ethics that applies to all of its employees, including its principal executive officer, principal financial officer and principal accounting officer, and the Board. A copy of this code is available in the Company’s employee handbook and under the “About Us – Code of Conduct” section of the Company’s website at www.avalon-globocare.com. In addition, the Company intends to post on its website all disclosures that are required by law or the listing standards of our applicable trading market concerning any amendments to, or waivers from, any provision of the code. The reference to the Company’s website address does not constitute incorporation by reference of the information contained at or available through the Company’s website, and you should not consider it to be a part of this proxy statement.
Anti-Hedging Policy
Under the terms of our insider trading policy, we prohibit each officer, director and employee, and each of their family members and controlled entities, from engaging in certain forms of hedging or monetization transactions. Such transactions include those, such as zero-cost collars and forward sale contracts, that would allow them to lock in much of the value of their stock holdings, often in exchange for all or part of the potential for upside appreciation in the stock, and to continue to own the covered securities but without the full risks and rewards of ownership.
99
Indemnification of Directors and Officers
The Company’s directors and executive officers are indemnified as provided by the Delaware law and the Company’s Bylaws. These provisions state that the Company’s directors may cause the Company to indemnify a director or former director against all costs, charges and expenses, including an amount paid to settle an action or satisfy a judgment, actually and reasonably incurred by him or her as a result of him or her acting as a director. The indemnification of costs can include an amount paid to settle an action or satisfy a judgment. Such indemnification is at the discretion of the Company’s board of directors and is subject to the Securities and Exchange Commission’s policy regarding indemnification.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers or persons controlling the Company pursuant to the foregoing provisions, or otherwise, the Company has been advised that in the opinion of the Securities and Exchange Commission, such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
COMPENSATION OF DIRECTORS
The following table sets forth compensation information for the Company’s directors for the year ended December 31, 2020.
| Name | Fees Earned or Paid in Cash $ | Stock Awards $ | Option Awards $ | Non-equity Incentive Plan Compensation $ | Change in Pension Value and Non- Qualified Deferred Compensation Earnings | All Other Compensation $ | Total $ | |||||||||||||||||||||
| Yue (Charles) Li (1) | 60,000 | - | 116,808 | - | - | - | 176,808 | |||||||||||||||||||||
| Yancen Lu (2) | 70,000 | - | 116,808 | - | - | - | 186,808 | |||||||||||||||||||||
| Wilbert Tauzin (3) | - | - | 249,137 | - | - | - | 249,137 | |||||||||||||||||||||
| Wenzhao Lu | 100,000 | - | - | - | - | - | 100,000 | |||||||||||||||||||||
| David Jin | - | - | - | - | - | - | - | |||||||||||||||||||||
| Meng Li | - | - | - | - | - | - | - | |||||||||||||||||||||
| Steven Sanders (4) | 70,000 | - | 116,808 | - | - | - | 186,808 | |||||||||||||||||||||
| Tevi Troy (5) | 60,000 | - | 116,808 | - | - | - | 176,808 | |||||||||||||||||||||
| William Stilley (6) | 70,000 | - | 116,808 | - | - | - | 186,808 | |||||||||||||||||||||
| (1) | Mr. Li’s 2020 compensation consisted of cash of $60,000 and 80,000 options vested and valued at $116,808. |
| (2) | Mr. Lu’s 2020 compensation consisted of cash of $70,000 and 80,000 options vested and valued at $116,808. |
| (3) | Mr. Tauzin’s 2020 compensation consisted of 200,000 options vested and valued at $249,137. |
| (4) | Mr. Sanders’s 2020 compensation consisted of cash of $70,000 and 80,000 options vested and valued at $116,808. |
| (5) | Mr. Troy’s 2020 compensation consisted of cash of $60,000 and 80,000 options vested and valued at $116,808. |
| (6) | Mr. Stilley’s 2020 compensation consisted of cash of $70,000 and 80,000 options vested and valued at $116,808. |
On February 19, 2020, the Board of Directors of the Company approved an increase in the number of shares of common stock to be acquired pursuant to option grants for all independent Directors from 50,000 shares to 80,000 shares annually going forward, which shall vest at the rate of 20,000 shares under such option per quarter.
REPORT OF THE AUDIT COMMITTEE OF THE BOARD OF DIRECTORS
The Audit Committee, on behalf of the Company’s Board of Directors, serves as an independent and objective party to monitor and provide general oversight of the integrity of the Company’s consolidated financial statements, the Company’s independent registered public accounting firm’s qualifications and independence, the performance of the Company’s independent registered public accounting firm and the Company’s standards of business conduct. The Audit Committee performs these oversight responsibilities in accordance with its Audit Committee Charter.
100
The Company’s management is responsible for preparing our consolidated financial statements and managing the Company’s financial reporting process. The Company’s independent registered public accounting firm is responsible for expressing an opinion on the conformity of the Company’s audited consolidated financial statements to generally accepted accounting principles in the United States of America. The Audit Committee met with the Company’s independent registered public accounting firm, with and without management present, to discuss the results of their examinations and the overall quality of the Company’s financial reporting.
In this context, the Audit Committee reviewed and discussed the Company’s audited consolidated financial statements for the year ended December 31, 2020 with management and with the Company’s independent registered public accounting firm. The Audit Committee has discussed with the Company’s independent registered public accounting firm the matters required to be discussed by the statement on PCAOB AS 16 (Communications with Audit Committees), as adopted by the Public Company Accounting Oversight Board in Rule 3200T, which includes, among other items, matters related to the conduct of the audit of the Company’s annual consolidated financial statements.
The Audit Committee has received the written disclosures and the letter from the independent registered public accounting firm required by applicable requirements of the Public Company Accounting Oversight Board regarding such independent registered public accounting firm’s communications with the Audit Committee concerning independence, and has discussed with the independent registered public accounting firm its independence from the Company and its management.
Based on its review of the audited financial statements and the various discussions noted above, the Audit Committee recommended to the Company’s Board of Directors that the Company’s audited consolidated financial statements be included in the Company’s Annual Report on Form 10-K for the year ended December 31, 2020.
Respectfully submitted by the Audit Committee,
William Stilley (Chairman)
Yancen Lu
Steve Sanders
The foregoing Audit Committee Report does not constitute soliciting material and shall not be deemed filed or incorporated by reference into any other filing of the Company under the Securities Act of 1933, as amended (the “Securities Act”), or the Exchange Act, except to the extent the Company specifically incorporate this Audit Committee Report by reference therein.
INFORMATION ABOUT THE EXECUTIVE OFFICERS
The executive officers are appointed annually by the Company’s Board of Directors and hold office until their successors are elected and duly qualified unless otherwise specified in an individual’s employment agreement. The current executive officers of the Company, and their ages as of June 30, 2021 are as follows:
| Name | Age | Position | ||
| David Jin, MD, PHD | 53 | Chief Executive Officer and President | ||
| Meng Li | 43 | Chief Operating Officer and Secretary | ||
| Luisa Ingargiola | 53 | Chief Financial Officer |
Biographical information regarding our executive officers as of June 30, 2021 is set forth below:
David Jin, Chief Executive Officer, President and Director
See Dr. Jin’s biography set forth in “Proposal No. 1 — The Director Election Proposal.”
101
Meng Li, Chief Operating Officer and Secretary
See Ms. Li’s biography set forth in “Proposal No. 1 — The Director Election Proposal.”
Luisa Ingargiola, Chief Financial Officer
Luisa Ingargiola is the Company’s Chief Financial Officer. Ms Ingargiola has significant experience serving as Chief Financial Officer or Audit Chair for multiple NASDAQ and NYSE companies. She currently serves as Director and Audit Chair for several public companies including ElectraMeccanica (NASDAQ:SOLO), AgEagle (NYSE:UAVS), Siyata Mobile (NASDAQ:SYTA) and Progress Acquisition Corporation (NASDAQ:PGRWU). From 2007 through 2016, Ms. Ingargiola served as the Chief Financial Officer and then Director at MagneGas Corporation (Nasdaq: MNGA). Prior to 2007, Ms. Ingargiola held various roles as Budget Director and Investment Analyst in several private companies. Ms. Ingargiola graduated in 1989 from Boston University with a Bachelor’s degree in Business Administration and a concentration in Finance. In 1996, she received her MBA in Health Administration from the University of South Florida. Ms. Ingargiola is qualified to serve as a Chief Financial Officer because of her extensive knowledge corporate governance, regulatory requirements, executive leadership and knowledge of, and experience in, financing and M&A transactions.
EXECUTIVE COMPENSATION
Executive Officers’ Compensation
The following table sets forth information concerning the annual and long-term compensation earned by or paid to the Company’s Chief Executive Officer and to other persons who served as executive officers as at and/or during the fiscal year ended December 31, 2020 or who earned compensation exceeding $100,000 during fiscal year 2020 (the “named executive officers”), for services as executive officers for the last two fiscal years.
Summary Compensation Table
| Name and Principal Position | Fiscal Year | Salary | Stock Award | Option Awards | Non-Equity Incentive Plan Compensation | Change
in Pension Value and Non- Qualified Deferred Compensation Earnings | All Other Compensation | Total | |||||||||||||||||||||||
| ($) | ($) | ($) | ($) | ($) | ($) | ($) | |||||||||||||||||||||||||
| Dr. David Jin | 2020 | 360,000 | - | 642,584 | - | - | - | 1,002,584 | |||||||||||||||||||||||
| CEO | 2019 | 540,000 | - | 394,722 | - | - | - | 934,722 | |||||||||||||||||||||||
| Luisa Ingargiola | 2020 | 350,000 | - | 712,028 | - | - | - | 1,062,028 | |||||||||||||||||||||||
| CFO | 2019 | 490,000 | - | 833,333 | - | - | - | 1,323,333 | |||||||||||||||||||||||
| Meng Li | 2020 | 340,000 | - | 481,942 | - | - | - | 821,942 | |||||||||||||||||||||||
| COO and Secretary | 2019 | 374,000 | - | 394,722 | - | - | - | 768,722 | |||||||||||||||||||||||
102
Employment Agreements
David Jin
On December 1, 2016, the Company entered into an Executive Employment Agreement with David Jin, the Company’s CEO and President. Pursuant to the agreement, Mr. Jin will be employed as President and Chief Executive Officer of the Company until November 30, 2017 unless earlier terminated pursuant to the terms of the agreement. During the term of the agreement, Mr. Jin will be entitled to a base salary at the annualized rate of $200,000 and will be eligible for a discretionary performance bonus, equity awards and to participate in employee benefits plans as the Company may institute from time to time at the discretion of the Company’s Board of Directors. Pursuant to the agreement, Mr. Jin may be terminated for “cause” as defined and Mr. Jin may resign for “good reason” as defined. In the event Mr. Jin is terminated without cause or resigns for good reason, the Company will be required to pay Mr. Jin all accrued salary and bonuses, reimbursement for all business expenses and Mr. Jin’s salary for one year. In the event Mr. Jin is terminated with cause, resigns without good reason, dies or is disabled, the Company will be required to pay Mr. Jin all accrued salary and bonuses and reimbursement for all business expenses. Under the agreement Mr. Jin is subject to confidentiality, non-compete and non-solicitation restrictions.
On January 3, 2019, the Company entered into a Letter Agreement with Dr. Jin, pursuant to which his annual base salary set forth in his employment agreement was increased to $360,000 effective January 1, 2019. Further, the Company agreed to grant Dr. Jin stock options to acquire 150,000 shares of common stock at an exercise price of $2.00 per share.
On February 20, 2020, the Company entered into a Letter Agreement with Dr. Jin pursuant to which the term of Dr. Jin’s Executive Employment Agreement entered between the Company and Dr. Jin dated December 1, 2016 was extended an additional three years and granted Dr. Jin a Stock Option to acquire 400,000 shares of common stock at an exercise price of $1.52 per share for a period of ten years.
Meng Li
On January 11, 2017, Avalon Shanghai entered into an Executive Employment Agreement with Meng Li, the Company’s COO and Secretary. Pursuant to the agreement, Ms. Li will be employed as Chief Operating Officer and President of Avalon Shanghai through November 30, 2019, unless earlier terminated pursuant to the terms of the agreement. During the term of the agreement, Ms. Li will be entitled to a base salary at the annualized rate of $100,000 and will be eligible for a discretionary performance bonus, equity awards and to participate in employee benefits plans as the Avalon Shanghai may institute from time to time at the discretion of its Board of Directors. Pursuant to the agreement, Ms. Li may be terminated for “cause” as defined and Ms. Li may resign for “good reason” as defined. In the event Ms. Li is terminated without cause or resigns for good reason, Avalon Shanghai will be required to pay Ms. Li all accrued salary and bonuses, reimbursement for all business expenses and Ms. Li’s salary for one year. In the event Ms. Li is terminated with cause, resigns without good reason, dies or is disabled, Avalon Shanghai will be required to pay Ms. Li all accrued salary and bonuses and reimbursement for all business expenses. Under the agreement Ms. Li is subject to confidentiality, non-compete and non-solicitation restrictions.
On January 3, 2019, the Company entered into a Letter Agreement with Ms. Li, pursuant to which her annual base salary set forth in her employment agreement was increased to $340,000 effective January 1, 2019. Further, the Company agreed to grant Ms. Li stock options to acquire 150,000 shares of common stock at an exercise price of $2.00 per share.
On February 20, 2020, the Company entered into a Letter Agreement with Meng Li pursuant to which the term of Ms. Li’s Executive Employment Agreement entered between the Company’ subsidiary and Ms. Li dated January 11, 2017 was extended an additional three years and granted Ms. Li a Stock Option to acquire 300,000 shares of common stock at an exercise price of $1.52 per share for a period of ten years.
Luisa Ingargiola
On February 21, 2017, Ms. Ingargiola and the Company entered into an Executive Retention Agreement effective February 9, 2017 pursuant to which Ms. Ingargiola agreed to serve as Chief Financial Officer in consideration of an annual salary of $200,000 to be increased to $225,000 on the 60-day anniversary. The Company has agreed to provide a bonus of 50% of her base salary upon the Company timely filing its annual report on Form 10-K for the year ended December 31, 2017 and the Company raising gross proceeds of $20 million in debt and/or equity capital and a bonus of 100% of her base salary upon the Company achieving (i) any merger or sale of the Company or its assets, (ii) the Company achieving adjusted EBITDA of $10 million in a fiscal year, (iii) the Company achieving a listing on a national exchange and then or subsequently raising gross proceeds in the amount of $10 million. The Company also granted Ms. Ingargiola a Stock Option to acquire two million shares of common stock of the Company at an exercise price of $0.50 per share for a period of ten years. The Stock Options vest in 36 equal tranches commencing on the grant date. The Company and Ms. Ingargiola also entered into an Indemnification Agreement.
103
The employment of Ms. Ingargiola is at will and may be terminated at any time, with or without formal cause. Pursuant to the terms of executive retention agreement with Ms. Ingargiola, the Company has agreed to provide specified severance and bonus amounts and to accelerate the vesting on their equity awards upon termination upon a change of control or an involuntary termination, as each term is defined in the agreements.
In the event of a termination upon a change of control, Ms. Ingargiola is entitled to receive an amount equal to 12 months of her base salary and the target bonus then in effect for the executive officer for the year in which such termination occurs, such bonus payment to be pro-rated to reflect the full number of months the executive remained in the Company’s employ. In addition, the vesting on any stock option held by the executive officer will be accelerated in full. At the election of the executive officer, the Company will also continue to provide health related employee insurance coverage for twelve months, at the Company’s expense.
In the event of an involuntary termination, Ms. Ingargiola is entitled to receive an amount equal to six months of her base salary and the target bonus then in effect for the executive officer for the six months in which such termination occurs, such bonus payment to be pro-rated to reflect the full number of months the executive remained in the Company’s employ. Such payment will be increased to 12 months upon the one-year anniversary of the retention agreement. In addition, the vesting on any stock option held by the executive officer will be accelerated in full. At the election of the executive officer, the Company will also continue to provide health related employee insurance coverage for twelve months, at the Company’s expense.
On January 3, 2019, the Company entered into a Letter Agreement with Ms. Ingargiola, pursuant to which her annual base salary set forth in her employment agreement was increased to $350,000 effective January 1, 2019.
On February 20, 2020, the Company entered into a Letter Agreement with Ms. Ingargiola granting Ms. Ingargiola a Stock Option to acquire 400,000 shares of common stock at an exercise price of $1.52 per share for a period of ten years.
Yu Zhou
On October 25, 2017, Dr. Yu Zhou and Genexosome entered into an Executive Retention Agreement pursuant to which Dr. Zhou agreed to serve as Co-Chief Executive Officer in consideration of an annual salary of $ 160,000. Dr. Zhou and Genexosome also entered into an Invention Assignment, Confidentiality, Non-Compete and Non-Solicit Agreement. On August 14, 2019, Genexosome terminated Yu Zhou as Co-Chief Executive Officer. In addition, Dr. Zhou’s Executive Retention Agreement was also terminated and he was not elected to serve as a director for the year ended 2020.
Outstanding Equity Awards
The following table sets forth information with respect to the outstanding equity awards of our principal executive officers and principal financial officer during 2020, and each person who served as an executive officer of the Company as of December 31, 2020:
| Outstanding Equity Awards | ||||||||||||||||||||||||||||||||||||
| Option Awards | Stock Awards | |||||||||||||||||||||||||||||||||||
| Name and principal position | Number of securities underlying unexercised options Exercisable (#) | Number of securities underlying unexercised options Unexercisable (#) | Equity incentive plan awards: Number of securities underlying unexercised options (#) | Options exercise price ($) | Option expiration Date | Number of shares or units of stock that have not vested (#) | Market value of shares or units of stock that have not vested ($) | Equity incentive plan awards: Number of unearned shares, units or other rights that have not vested (#) | Equity incentive plan awards: Market or payout value of unearned shares, units or other rights that have not vested ($) | |||||||||||||||||||||||||||
| Luisa Ingargiola, CFO | 455,556 | - | 455,556 | 0.50 and 1.52 | 2/8/2027 and 2/18/2030 | - | - | - | - | |||||||||||||||||||||||||||
| David Jin, CEO | 400,000 | - | 400,000 | 1.52 | 2/18/2030 | - | - | - | - | |||||||||||||||||||||||||||
| Meng Li, COO and Secretary | 300,000 | - | 300,000 | 1.52 | 2/18/2030 | - | - | - | - | |||||||||||||||||||||||||||
104
SECTION 16(A) BENEFICIAL OWNERSHIP REPORTING COMPLIANCE
Section 16(a) of the Exchange Act requires our directors and executive officers and beneficial owners of more than 10% of our outstanding common stock (collectively, “Insiders”) to file reports with the SEC disclosing direct and indirect ownership of our common stock and changes in such ownership. The rules of the SEC require Insiders to provide us with copies of all Section 16(a) reports filed with the SEC. Based solely upon a review of copies of Section 16(a) reports received by us, and written representations that no additional reports were required to be filed with the SEC, we believe that our Insiders have timely filed all Section 16(a) reports during the 2020 fiscal year.
105
AVALON MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
All references in this section to “Avalon,” the “Company,” “we,” “us,” or “our” mean Avalon GloboCare Corp. unless we state otherwise or the context otherwise indicates.
This Management’s Discussion and Analysis (“MD&A”) contains “forward-looking statements”, within the meaning of the Private Securities Litigation Reform Act of 1995, which represent our projections, estimates, expectations, or beliefs concerning, among other things, financial items that relate to management’s future plans or objectives or to our future economic and financial performance. In some cases, you can identify these statements by terminology such as “may”, “should”, “plans”, “believe”, “will”, “anticipate”, “estimate”, “expect” “project”, or “intend”, including their opposites or similar phrases or expressions. You should be aware that these statements are projections or estimates as to future events and are subject to a number of factors that may tend to influence the accuracy of the statements. These forward-looking statements should not be regarded as a representation by us or any other person that our events or plans will be achieved. You should not unduly rely on these forward-looking statements, which speak only as of the date of this report. Except as may be required under applicable securities laws, we undertake no obligation to publicly revise any forward-looking statement to reflect circumstances or events after the date of this report or to reflect the occurrence of unanticipated events.
You should review the factors and risks we describe under “Risk Factors” herein and in our Annual Report on Form 10-K for the year ended December 31, 2020, which Annual Report is being sent to stockholders together with this proxy statement. Actual results may differ materially from any forward-looking statement.
Impact of COVID-19 on Our Operations, Financial Condition, Liquidity and Results of Operations
Although the COVID-19 vaccines have generally been introduced to the public, the ultimate impact of the COVID-19 pandemic on our operations is unknown and will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration of the COVID-19 outbreak, new information which may emerge concerning the severity of the COVID-19 pandemic, a significant increase in new and variant strains of COVID-19 cases, availability and effectiveness of COVID-19 vaccines and therapeutics, the level of acceptance of the vaccine by the general population and any additional preventative and protective actions that governments, or us, may determine are needed.
The occurrence of COVID-19 pandemic had negative impact on our operations. Some of the universities and laboratories with which we collaborate were temporarily closed. Our general development operations have continued during the COVID-19 pandemic and we have not had significant disruption. However, we are uncertain if the COVID-19 pandemic will impact future operations at our laboratory, or our ability to collaborate with other laboratories and universities. In addition, we are unsure if the COVID-19 pandemic will impact future clinical trials. Given the dynamic nature of these circumstances, the duration of business disruption and reduced traffic, the related financial effect cannot be reasonably estimated at this time but is expected to adversely impact the Company’s business for the rest of 2021.
Assuming the Acquisition and the Equity Financing do not close, we have limited cash available to fund planned operations and although we have other sources of capital described below under “Liquidity and Capital Resources,” management continues to pursue various financing alternatives to fund our operations so we can continue as a going concern. However, the COVID-19 pandemic has created significant economic uncertainty and volatility in the credit and capital markets. Management plans to secure the necessary financing through the issue of new equity and/or the entering into of strategic partnership arrangements but the ultimate impact of the COVID-19 pandemic on our ability to raise additional capital is unknown and will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration of the COVID-19 outbreak and new information which may emerge concerning the severity of the COVID-19 pandemic. We may not be able to raise sufficient additional capital and may tailor our operations based on the amount of funding we are able to raise in the future. Nevertheless, there is no assurance that these initiatives will be successful. Further, there is no assurance that capital available to us in any future financing will be on acceptable terms.
106
Overview
The Company is a clinical-stage, vertically integrated, leading CellTech bio-developer dedicated to advancing and empowering innovative, transformative immune effector cell therapy, exosome technology, as well as COVID-19 related diagnostics and therapeutics. The Company also provides strategic advisory and outsourcing services to facilitate and enhance its clients' growth and development, as well as competitiveness in healthcare and CellTech industry markets. Through its subsidiary structure with unique integration of verticals from innovative R&D to automated bioproduction and accelerated clinical development, the Company is establishing a leading role in the fields of cellular immunotherapy (including CAR-T/NK), exosome technology (ACTEX™), and regenerative therapeutics. Avalon achieves and fosters seamless integration of unique verticals to bridge and accelerate innovative research, bio-process development, clinical programs and product commercialization. Avalon’s upstream innovative research includes:
| ● | Development of Avalon Clinical-grade Tissue-specific Exosome (“ACTEX™”). | |
| ● | Novel therapeutic and diagnostic targets development utilizing QTY-code protein design technology with Massachusetts Institute of Technology (MIT) including using the QTY code protein design technology for development of a hemofiltration device to treat Cytokine Storm. | |
| ● | Strategic partnership with the University of Natural Resources and Life Sciences (BOKU) in Vienna, Austria to develop an S-layer vaccine that can be administered by an intranasal or oral route against SARS-CoV-2, the novel coronavirus that causes COVID-19 disease. |
Avalon’s midstream bio-processing and bio-production facility is located in Nanjing, China with state-of-the-art, automated GMP and QC/QA infrastructure for standardized bio-manufacturing of clinical-grade cellular products involved in our clinical programs in immune effector cell therapy, regenerative therapeutics, as well as bio-banking. As a result of the COVID pandemic, the operation of this facility has not been at full capacity. However, the Company expects to slowly increase operations during 2021.
Avalon’s downstream medical team and facility consists of top-rated affiliated hospital network and experts specialized in hematology, oncology, cellular immunotherapy, hematopoietic stem/progenitor cell transplant, as well as regenerative therapeutics. Our major clinical programs include:
| ● | AVA-001: Avalon has initiated its first-in-human clinical trial of CD19 CAR-T candidate, AVA-001 in August 2019 at the Hebei Yanda Lu Daopei Hospital and Beijing Lu Daopei Hospital in China (the world’s single largest CAR-T treatment network with over 600 patients being treated with CAR-T) for the indication of relapsed/refractory B-cell acute lymphoblastic leukemia and non-Hodgkin Lymphoma. The AVA-001 candidate (co-developed with China Immunotech Co. Ltd) is characterized by the utilization of 4-1BB (CD137) co-stimulatory signaling pathway, conferring a strong anti-cancer activity during pre-clinical study. It also features a shorter bio-manufacturing time which leads to the advantage of prompt treatment to patients where timing is important related hematologic malignancies. Avalon has successfully completed the first-in-human clinical trial of its AVA-001 anti-CD19 CAR-T cell therapy as a bridge to allogeneic bone marrow transplantation for patients with relapsed/refractory B-cell acute lymphoblastic leukemia at the Lu Daopei Hospital (registered clinical trial number NCT03952923) with excellent efficacy (90% complete remission rate) and minimal adverse side effects. Avalon is currently expanding the patient recruitment for AVA-001 to include relapsed/refractory non-Hodgkin lymphoma patients. |
| ● | ACTEX™: Stem cell-derived Avalon Clinical-grade Tissue-specific Exosomes (ACTEX™) is one of the core technology platforms that has been co-developed by Avalon GloboCare and Weill Cornell Medicine. The Company formed a strategic partnership with HydroPeptide, LLC, a leading epigenetics skin care company, to engage in co-development and commercialization of a series of clinical-grade, exosome-based cosmeceutical and orthopedic products. As part of this agreement, the Company signed a three-way Material Transfer Agreement between Avalon GloboCare, HydroPeptide and Weill Cornell Medicine. |
| ● | FLASH-CAR™: The Company advanced its next generation immune cell therapy using RNA-based, non-viral FLASH-CAR™ technology co-developed with the Company’s strategic partner Arbele Limited. The adaptable FLASH-CAR™ platform can be used to create personalized cell therapy from a patient’s own cells, as well as off-the-shelf cell therapy from a universal donor. Our leading candidate, AVA-011, is currently at process development stage to generate clinical-grade cell-therapy products for subsequent clinical studies. |
| ● | AVA-Trap™: Avalon’s AVA-Trap™ therapeutic program plans to enter animal model testing followed by expedited clinical studies with the goal of providing an effective therapeutic option to combat COVID-19 and other life-threatening conditions involving cytokine storms. The Company initiated a sponsored research and co-development project with Massachusetts Institute of Technology (MIT) led by Professor Shuguang Zhang as Principal Investigator in May 2019. Using the unique QTY code protein design platform, six water-soluble variant cytokine receptors have been successfully designed and tested to show binding affinity to the respective cytokines. |
107
We provide medical related consulting services in advanced areas of immunotherapy and second opinion/referral services through our wholly-owned subsidiary Avalon (Shanghai) Healthcare Technology Co., Ltd., or Avalon Shanghai. We also own and operate rental commercial real property in New Jersey, where we are headquartered.
During the first quarter of 2021, we did not have any revenue from medical related consulting services. Although we maintain close working relationships with our related parties, the consulting agreements with our related parties expired as of December 31, 2020. There was no order from related party and third party customers in the three months ended March 31, 2021. Currently, we are negotiating with our potential customers and consulting services agreements are not finalized.
The value of the Renminbi (“RMB”), the main currency used in China, fluctuates and is affected by, among other things, changes in China’s political and economic conditions. The conversion of RMB into foreign currencies such as the U.S. dollar have generally been based on rates set by the People’s Bank of China, which are set daily based on the previous day’s interbank foreign exchange market rates and current exchange rates on the world financial markets.
Sen Lang Acquisition and Equity Financing
On June 13, 2021, the Company entered into a Share Purchase Agreement (the “Purchase Agreement”), by and among the Company, Lonlon Biotech Ltd., a company incorporated in the British Virgin Islands (“BVI”) (“Sen Lang”), the holders of the share capital of Sen Lang (the “Sen Lang Shareholders”), the ultimate beneficial owners of the Sen Lang Shareholders (the “Sen Lang Beneficial Shareholders” and, together with the Sen Lang Shareholders, the “Sen Lang Owners”) and a representative of the Sen Lang Owners (the “Sen Lang Representative”). Pursuant to the Purchase Agreement, subject to the satisfaction of the conditions to closing therein, including approval by the Avalon stockholders pursuant to the rules of the Nasdaq Stock Market (“Nasdaq”), Avalon agreed to purchase (the “Acquisition”) all of the issued and outstanding share capital of Sen Lang (the “Sen Lang Shares”).
Sen Lang, through a “variable interest entity” structure (described in more detail below under “VIE Structure”) of contractual rights held by its wholly-owned subsidiary Beijing Langlang Runfeng Biotechnology Co., Ltd., a wholly foreign owned enterprise with limited liability organized and existing under the laws of the People’s Republic of China (the “PRC”) (the “PRC Subsidiary”), has full economic benefit and management control over, and is consolidated for accounting purposes with, Senlang Biotechnology Co. Ltd., a PRC domestic company with limited liability organized and existing under the laws of the PRC (the “OpCo” or “SenlangBio”). SenlangBio is mainly engaged in the business of research and development in relation to CAR-T cell therapy, immune cell therapy and related drug development. SenlangBio is owned 100% by certain of the Sen Lang Beneficial Shareholders. A wholly-owned subsidiary of SenlangBio, Shijiazhuang Senlang Medical Laboratory Co., Ltd., a company with limited liability organized and existing under the laws of the PRC (“SenlangBio Clinical Laboratory”) is engaged in the business of testing of immunology, serology and molecular genetics specialties for patients, including hematology-tumor diagnostics and testing prior to clinical trials for cell therapy. For a more detailed discussion of the business of SenlangBio and SenlangBio Clinical Laboratory, please see the section entitled “Information about SenlangBio.”
The purchase price being paid by Avalon to the Sen Lang Shareholders under the Purchase Agreement for the Sen Lang Shares is an aggregate of 81 million shares (the “Acquisition Shares”) of the common stock, par value US$0.0001 per share, of Avalon (the “Avalon Common Stock”). Ten percent (10%), or 8.1 million, of such shares will be held in escrow for 12 months following the closing to satisfy any indemnification obligations of the Sen Lang Shareholders under the Share Purchase Agreement. In addition, at the closing of the Acquisition, it is expected that Dr. Jianqiang Li, scientific founder and CSO of SenlangBio, will join the board of the Company, and Dr. Li will also be appointed as Chief Technology Officer of the Company. The Acquisition Shares will not be registered under the Securities Act of 1933, as amended (the “Securities Act”) and, therefore, will be restricted securities under Rule 144 under the Securities Act for six months or longer after the closing of the Acquisition, subject to “affiliate” status with the Company under the Securities Act.
In connection with the Acquisition, on June 13, 2021, an institutional investor (the “Investor”) entered into an agreement, as amended on June 24, 2021, with SenlangBio related to the purchase of registered capital of SenlangBio (the “OpCo Capital Increase Agreement”) pursuant to which the Investor will acquire an aggregate of up to 13.5% of the equity ownership of SenlangBio for an aggregate purchase price (the “Subscription Amount) of approximately US$30,000,000 (represented by an actual investment of RMB200,000,000) (the “Equity Financing”), which funds will be invested in SenlangBio in three equal installments of approximately US$10,000,000, at a fixed price, the first to be upon the closing of the Acquisition, the second to be within three months after the closing and the third to be within six months after the closing. In addition, pursuant to a Securities Exchange Agreement, as amended on June 24, 2021 (the “Exchange Agreement”), by and among the Company, Sen Lang, SenlangBio and the Investor, dated June 13, 2021, the Investor shall have the right, exercisable between the six-month and five year-anniversaries of the respective initial closing and installment closings, to elect to exchange, from time to time, all or part of its then-owned equity ownership of SenlangBio for shares (the “Exchange Shares”) of Avalon Common Stock at a fixed exchange price of US$1.21 per share of Avalon Common Stock, which was the market price of the Avalon Common Stock as of the date of the Exchange Agreement under Nasdaq rules. In addition, the Exchange Agreement provides that the Investor may only exchange up to 10% of its total investment amount in any 30 day period.
108
Going Concern
The Company is a clinical-stage, vertically integrated, leading CellTech bio-developer dedicated to advancing and empowering innovative, transformative immune effector cell therapy, exosome technology, as well as COVID-19 related diagnostics and therapeutics. The Company also provides strategic advisory and outsourcing services to facilitate and enhance its clients' growth and development, as well as competitiveness in healthcare and CellTech industry markets. Through its subsidiary structure with unique integration of verticals from innovative R&D to automated bioproduction and accelerated clinical development, the Company is establishing a leading role in the fields of cellular immunotherapy (including CAR-T/NK), exosome technology (ACTEX™), and regenerative therapeutics.
In addition, the Company owns commercial real estate that houses its headquarters in Freehold, New Jersey and provides outsourced, customized international healthcare services to the rapidly changing health care industry primarily focused in the People’s Republic of China. The Company did not generate any revenue from medical related consulting services segment during the three months ended March 31, 2021. These condensed consolidated financial statements have been prepared assuming that the Company will continue as a going concern, which contemplates, among other things, the realization of assets and the satisfaction of liabilities in the normal course of business.
As reflected in the accompanying condensed consolidated financial statements, the Company had working capital deficit of $1,059,606 as of March 31, 2021 and has incurred recurring net loss and generated negative cash flow from operating activities of $2,367,118 and $1,515,525 for the three months ended March 31, 2021, respectively. The Company has a limited operating history and its continued growth is dependent upon the continuation of providing medical consulting services to its only few clients who are related parties and generating rental revenue from its income-producing real estate property in New Jersey; hence generating revenues, and obtaining additional financing to fund future obligations and pay liabilities arising from normal business operations. In addition, the current cash balance cannot be projected to cover the operating expenses for the next twelve months from the release date of this report. These matters raise substantial doubt about the Company’s ability to continue as a going concern. The ability of the Company to continue as a going concern is dependent on the Company’s ability to raise additional capital, implement its business plan, and generate significant revenues. There are no assurances that the Company will be successful in its efforts to generate significant revenues, maintain sufficient cash balance or report profitable operations or to continue as a going concern. The Company plans on raising capital through the sale of equity to implement its business plan. However, there is no assurance these plans will be realized and that any additional financings will be available to the Company on satisfactory terms and conditions, if any.
The occurrence of an uncontrollable event such as the COVID-19 pandemic had negatively impact on the Company’s operations. Some tenants have delayed on rent payment. Our general development operations have continued during the COVID-19 pandemic and we have not had significant disruption. However, we are uncertain if the COVID-19 pandemic will impact future operations at our laboratory, or our ability to collaborate with other laboratories and universities. In addition, we are unsure if the COVID-19 pandemic will impact future clinical trials. Given the dynamic nature of these circumstances, the duration of business disruption and reduced traffic, the related financial effect cannot be reasonably estimated at this time but is expected to adversely impact the Company’s business for the rest of 2021.
The accompanying condensed consolidated financial statements do not include any adjustments related to the recoverability or classification of asset-carrying amounts or the amounts and classification of liabilities that may result should the Company be unable to continue as a going concern.
Critical Accounting Policies
Use of Estimates
Our discussion and analysis of our financial condition and results of operations are based upon our condensed consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these condensed consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. We continually evaluate our estimates, including those related to the useful life of property and equipment and investment in real estate, assumptions used in assessing impairment of long-term assets, valuation of deferred tax assets and the associated valuation allowances, and valuation of stock-based compensation.
109
We base our estimates on historical experience and on various other assumptions that we believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Any future changes to these estimates and assumptions could cause a material change to our reported amounts of revenues, expenses, assets and liabilities. Actual results may differ from these estimates under different assumptions or conditions.
Revenue Recognition
We recognize revenue under Accounting Standards Codification (“ASC”) Topic 606, Revenue from Contracts with Customers (“ASC 606”). The core principle of the revenue standard is that a company should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. The following five steps are applied to achieve that core principle:
| ● | Step 1: Identify the contract with the customer |
| ● | Step 2: Identify the performance obligations in the contract |
| ● | Step 3: Determine the transaction price |
| ● | Step 4: Allocate the transaction price to the performance obligations in the contract |
| ● | Step 5: Recognize revenue when the company satisfies a performance obligation |
In order to identify the performance obligations in a contract with a customer, a company must assess the promised goods or services in the contract and identify each promised goods or service that is distinct. A performance obligation meets ASC 606’s definition of a “distinct” goods or service (or bundle of goods or services) if both of the following criteria are met:
| ● | The customer can benefit from the goods or service either on its own or together with other resources that are readily available to the customer (i.e., the goods or service is capable of being distinct). |
| ● | The entity’s promise to transfer the goods or service to the customer is separately identifiable from other promises in the contract (i.e., the promise to transfer the goods or service is distinct within the context of the contract). |
If a goods or service is not distinct, the goods or service is combined with other promised goods or services until a bundle of goods or services is identified that is distinct.
The transaction price is the amount of consideration to which an entity expects to be entitled in exchange for transferring promised goods or services to a customer, excluding amounts collected on behalf of third parties (for example, some sales taxes). The consideration promised in a contract with a customer may include fixed amounts, variable amounts, or both. Variable consideration is included in the transaction price only to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved.
The transaction price is allocated to each performance obligation on a relative standalone selling price basis. The transaction price allocated to each performance obligation is recognized when that performance obligation is satisfied, at a point in time or over time as appropriate.
The Company’s revenues are derived from providing medial related consulting services for its’ related parties. Revenues related to its service offerings are recognized as the services are performed. Any payments received in advance of the performance of services are recorded as deferred revenue until such time as the services are performed.
We have determined that the ASC 606 does not apply to rental contracts, which are within the scope of other revenue recognition accounting standards.
Rental income from operating leases is recognized on a straight-line basis under the guidance of ASC 842. Lease payments under tenant leases are recognized on a straight-line basis over the term of the related leases. The cumulative difference between lease revenue recognized under the straight-line method and contractual lease payments are included in rent receivable on the condensed consolidated balance sheets.
We do not offer promotional payments, customer coupons, rebates or other cash redemption offers to our customers.
Income Taxes
We are governed by the income tax laws of China and the United States. Income taxes are accounted for pursuant to ASC 740 “Accounting for Income Taxes,” which is an asset and liability approach that requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in our financial statements or tax returns. The charge for taxes is based on the results for the period as adjusted for items, which are non-assessable or disallowed. It is calculated using tax rates that have been enacted or substantively enacted by the balance sheet date.
110
Deferred tax is accounted for using the balance sheet liability method in respect of temporary differences arising from differences between the carrying amount of assets and liabilities in the financial statements and the corresponding tax basis used in the computation of assessable tax profit. In principle, deferred tax liabilities are recognized for all taxable temporary differences, and deferred tax assets are recognized to the extent that it is probably that taxable profit will be available against which deductible temporary differences can be utilized.
Deferred tax is calculated using tax rates that are expected to apply to the period when the asset is realized or the liability is settled. Deferred tax is charged or credited in the income statement, except when it is related to items credited or charged directly to equity, in which case the deferred tax is changed to equity. Deferred tax assets and liabilities are offset when they related to income taxes levied by the same taxation authority and we intend to settle its current tax assets and liabilities on a net basis.
Recent Accounting Standards
For details of applicable new accounting standards, please, refer to Recent Accounting Standards in Note 3 of our condensed consolidated financial statements accompanying this report.
RESULTS OF OPERATIONS
Comparison of Results of Operations for the Three Months Ended March 31, 2021 and 2020
Revenues
For the three months ended March 31, 2021, we had real property rental revenue of $289,774, as compared to $296,956 for the three months ended March 31, 2020, a decrease of $7,182, or 2.4%. The slight decrease was primarily attributable to a tenant moved out in August 2020. We expect that our revenue from real property rent will remain in its current quarterly level with minimal increase in the near future.
Costs and Expenses
Real property operating expenses consist of property management fees, property insurance, real estate taxes, depreciation, repairs and maintenance fees, utilities and other expenses related to our rental properties.
For the three months ended March 31, 2021, our real property operating expenses amounted to $216,894, as compared to $254,501 for the three months ended March 31, 2020, a decrease of $37,607, or 14.8%. The decrease was mainly due to a decrease in repairs and maintenance fees of approximately $22,000, and a decrease in other miscellaneous items of approximately $16,000.
Real Property Operating Income
Our real property operating income for the three months ended March 31, 2021 was $72,880, representing an increase of $30,425, or 71.7%, as compared to $42,455 for the three months ended March 31, 2020. The increase was mainly attributable to the decrease in real property operating expenses as described above. We expect our real property operating income will remain in its current quarterly level with minimal increase in the near future.
Other Operating Expenses
For the three months ended March 31, 2021 and 2020, other operating expenses consisted of the following:
| Three Months Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| Professional fees | $ | 1,381,178 | $ | 1,553,698 | ||||
| Compensation and related benefits | 562,006 | 1,128,468 | ||||||
| Research and development | 213,188 | 275,402 | ||||||
| Directors and officers liability insurance premium | 81,141 | 58,013 | ||||||
| Travel and entertainment | 32,150 | 73,580 | ||||||
| Rent and related utilities | 22,627 | 22,741 | ||||||
| Advertising expenses | 8,823 | 70,903 | ||||||
| Other general and administrative | 75,355 | 81,842 | ||||||
| $ | 2,376,468 | $ | 3,264,647 | |||||
111
| ● | Professional fees primarily consisted of accounting fees, audit fees, legal service fees, consulting fees, investor relations service charges and other fees incurred for service related to being a public company. For the three months ended March 31, 2021, professional fees decreased by $172,520, or 11.1%, as compared to the three months ended March 31, 2020. The decrease was primarily attributable to a decrease in investor relations service fees of approximately $171,000 mainly due to the decrease in use of investor relations service providers, and a decrease in other miscellaneous items of approximately $1,000. We expect that our professional fees will remain in its current quarterly level with minimal increase in the near future. |
| ● | For the three months ended March 31, 2021, compensation and related benefits decreased by $566,462, or 50.2%, as compared to the three months ended March 31, 2020. The significant decrease was primarily attributable to a decrease in stock-based compensation of approximately $578,000 which reflected the value of options granted and vested to our management, offset by an increase in compensation and related benefits for other employees of approximately $12,000. We expect that our compensation and related benefits will remain in its current quarterly level with minimal increase in the near future. |
| ● | For the three months ended March 31, 2021, research and development expenses decreased by $62,214, or 22.6%, as compared to the three months ended March 31, 2020. The decrease was primarily attributable to our first project with Arbele was completed in 2020 and no further research and development project was incurred in 2021. We expect our research and development expenses will increase in the near future. |
| ● | For the three months ended March 31, 2021, Directors and Officers Liability Insurance premium increased by $23,128, or 39.9%, as compared to the three months ended March 31, 2020. The increase was mainly due to different insurance provider with different premium. |
| ● | For the three months ended March 31, 2021, travel and entertainment expense decreased by $41,430, or 56.3%, as compared to the three months ended March 31, 2020. The decrease was mainly due to decreased business travel activities and decreased entertainment expenditure resulting from COVID-19. The spread of COVID-19 has caused public health officials to recommend precautions to mitigate the spread of the virus, such as, cease traveling to non-essential jobs and curtail all unnecessary travel, and stay at home as much as possible. |
| ● | For the three months ended March 31, 2021, rent and related utilities expenses decreased by $114, or 0.5%, as compared to the three months ended March 31, 2020. |
| ● | For the three months ended March 31, 2021, advertising expenses decreased by $62,080 or 87.6% as compared to the three months ended March 31, 2020. The decrease was primarily due to decreased advertising activities incurred as a result of stricter control on corporation spending. We expect that our advertising expenses will increase in the near future. |
| ● | Other general and administrative expenses mainly consisted of NASDAQ listing fee, office supplies, and other miscellaneous items. For the three months ended March 31, 2021, other general and administrative expenses decreased by $6,487, or 7.9%, as compared to the three months ended March 31, 2020, reflecting our efforts at stricter controls on corporate expenditure. |
Loss from Operations
As a result of the foregoing, for the three months ended March 31, 2021, loss from operations amounted to $2,303,588, as compared to $3,222,192 for the three months ended March 31, 2020, a decrease of $918,604, or 28.5%.
Other Income (Expense)
Other income (expense) mainly includes interest expense and loss from equity method investment.
Other expense, net, totaled $63,530 for the three months ended March 31, 2021, as compared to $48,589 for the three months ended March 31, 2020, an increase of $14,941, or 30.7%, which was primarily attributable to an increase in loss from equity method investment of approximately $9,000, an increase in interest expense of approximately $3,000, and a decrease in miscellaneous income of approximately $3,000.
Income Taxes
We did not have any income taxes expense for the three months ended March 31, 2021 and 2020 since we incurred losses in these periods.
Net Loss
As a result of the factors described above, our net loss was $2,367,118 for the three months ended March 31, 2021, as compared to $3,270,781 for the three months ended March 31, 2020, a decrease of $903,663 or 27.6%.
Net Loss Attributable to Avalon GloboCare Corp. Common Shareholders
The net loss attributable to Avalon GloboCare Corp. common shareholders was $2,367,118 or $(0.03) per share (basic and diluted) for the three months ended March 31, 2021, as compared with $3,270,781, or $(0.04) per share (basic and diluted) for the three months ended March 31, 2020, a change of $903,663 or 27.6%.
112
Foreign Currency Translation Adjustment
Our reporting currency is the U.S. dollar. The functional currency of our parent company, AHS, Avalon RT 9, Genexosome, Avactis, and Exosome, is the U.S. dollar and the functional currency of Avalon Shanghai and Beijing Genexosome is the Chinese Renminbi (“RMB”). The financial statements of our subsidiaries whose functional currency is the RMB are translated to U.S. dollars using period end rates of exchange for assets and liabilities, average rate of exchange for revenues, costs, and expenses and cash flows, and at historical exchange rates for equity. Net gains and losses resulting from foreign exchange transactions are included in the results of operations. As a result of foreign currency translations, which are a non-cash adjustment, we reported a foreign currency translation loss of $2,722 and $22,066 for the three months ended March 31, 2021 and 2020, respectively. This non-cash loss had the effect of increasing our reported comprehensive loss.
Comprehensive Loss
As a result of our foreign currency translation adjustment, we had comprehensive loss of $2,369,840 and $3,292,847 for the three months ended March 31, 2021 and 2020, respectively.
Comparison of Results of Operations for the Years Ended December 31, 2020 and 2019
Revenues
For the year ended December 31, 2020, we had real property rental revenue of $1,206,854, as compared to $1,155,677 for the year ended December 31, 2019, an increase of $51,177, or 4.4%. The increase was primarily attributable to the increase of tenants in 2020. We expect that our revenue from real property rent will increase in the near future since our occupancy of our rental property increased in subsequent period.
For the year ended December 31, 2020, we had medical related consulting services revenue from related parties of $170,908, as compared to $355,544 for the year ended December 31, 2019, a decrease of $184,636, or 51.9%. The decrease was mainly attributable to the decreased demand for our consulting service from our related parties. We expect that our revenue from medical related consulting services will increase in the near future.
For the year ended December 31, 2020, we did not have any revenue from contract services through performing development services for hospitals and other customers and sales of developed products to hospitals and other customers. For the year ended December 31, 2019, we had revenue from contract services through performing development services for hospitals and other customers and sales of developed products to hospitals and other customers of $35,084. We have discontinued sales of our exosome isolation system product. However, we are actively developing other unrelated proprietary exosome related products for sale or licensure.
Costs and Expenses
Real property operating expenses consist of property management fees, property insurance, real estate taxes, depreciation, repairs and maintenance fees, utilities and other expenses related to our rental properties.
For the year ended December 31, 2020, our real property operating expenses amounted to $851,754, as compared to $818,662 for the year ended December 31, 2019, an increase of $33,092, or 4.0%. The increase was mainly due to an increase in property management fees of approximately $11,000, and an increase in other miscellaneous items of approximately $22,000.
Costs of medical related consulting services include the cost of labor and related benefits, travel expenses related to medical related consulting services, other related consulting costs, and other overhead costs.
For the year ended December 31, 2020, costs of medical related consulting services amounted to $135,805, as compared to $284,472 for the year ended December 31, 2019, a decrease of $148,667, or 52.3%. The decrease was mainly due to the decrease in medical related consulting services revenue.
Costs of development services and sales of developed products include inventory costs, materials and supplies costs, labor and related benefits, depreciation, other overhead costs and shipping and handling costs incurred.
For the year ended December 31, 2019, costs of development services for hospitals and other customers and sales of developed products to hospitals and other customers amounted to $103,258. We had neither revenue nor cost of revenue from this segment in the year ended December 31, 2020.
Real Property Operating Income
Our real property operating income for the year ended December 31, 2020 was $355,100, representing an increase of $18,085, or 5.4%, as compared to $337,015 for the year ended December 31, 2019. The increase was mainly attributable to the increase in rental revenue resulting from the increase of tenants as described above, offset by the increase in real property operating expenses. We expect our real property operating income will increase in the near future since our occupancy rate increased in subsequent period.
113
Gross Profit from Medical Related Consulting Services and Gross Margin
Gross profit from medical related consulting services for the year ended December 31, 2020 was $35,103, as compared to $71,072 for the year ended December 31, 2019, a change of $35,969, or 50.6%.
Gross margin increased to 20.5% for the year ended December 31, 2020 from gross margin of 20.0% for the year ended December 31, 2019. We estimate that our gross margin from medical related consulting services segment will remain at its current yearly level.
Gross Loss from Development Services and Sales of Developed Products and Gross Margin
We did not generate any gross profit from development services and sales of developed products in the year ended December 31, 2020. Our gross loss from development services and sales of developed products for the year ended December 31, 2019 was $68,174, with a gross margin of (194.3)%.
Other Operating Expenses
For the years ended December 31, 2020 and 2019, other operating expenses consisted of the following:
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Professional fees | $ | 6,553,009 | $ | 5,994,129 | ||||
| Compensation and related benefits | 4,156,150 | 8,743,691 | ||||||
| Research and development | 883,855 | 1,781,869 | ||||||
| Advertising expenses | 294,352 | 685,064 | ||||||
| Amortization | - | 245,678 | ||||||
| Travel and entertainment | 175,300 | 522,805 | ||||||
| Directors and officers liability insurance premium | 276,028 | 184,423 | ||||||
| Rent and related utilities | 92,370 | 91,033 | ||||||
| Other general and administrative | 413,158 | 458,440 | ||||||
| Impairment loss | - | 1,010,011 | ||||||
| $ | 12,844,222 | $ | 19,717,143 | |||||
| ● | Professional fees primarily consisted of accounting fees, audit fees, legal service fees, consulting fees, investor relations service charges and other fees incurred for service related to being a public company. For the year ended December 31, 2020, professional fees increased by $558,880, or 9.3%, as compared to the year ended December 31, 2019. The increase was primarily attributable to an increase in consulting fees of approximately $989,000 mainly due to the increase in stock-based consulting fees resulting from the increase in use of consulting service providers, an increase in accounting service charges of approximately $103,000 as a result of the increase in stock-based accounting fees and an increase in other miscellaneous items of approximately $49,000, offset by a decrease in legal service fees of approximately $582,000 as a result of decrease in use of legal service providers. We expect that our professional fees will remain in its current yearly level with minimal increase in the near future. |
| ● | For the year ended December 31, 2020, compensation and related benefits decreased by $4,587,541, or 52.5%, as compared to the year ended December 31, 2019. The significant decrease was primarily attributable to a decrease in stock-based compensation of approximately $4,133,000 which reflected the value of options granted and vested to our management, and a decrease in bonus for our three key officers of approximately $354,000, and a decrease in compensation and related benefits for other employees and directors of approximately $101,000, mainly due to the termination of employment in August 2019. We expect that our compensation and related benefits will remain in its current yearly level with minimal increase in the near future. | |
| ● | For the year ended December 31, 2020, research and development expenses decreased by $898,014, or 50.4%, as compared to the year ended December 31, 2019. Our first project with Arbele was completed in January 2020 and no further research and development project was incurred in 2020. Our research and development contract with Weill Cornell Medicine expired as of November 2019. Therefore, our research and development expenses decreased. We expect our research and development expenses will increase in the near future. |
| ● | For the year ended December 31, 2020, advertising expenses decreased by $390,712 or 57.0% as compared to the year ended December 31, 2019. The decrease was primarily due to decreased advertising activities incurred as a result of stricter control on corporation spending. We expect that our advertising expenses will continue to decrease in the near future. |
114
| ● | For the year ended December 31, 2020, amortization expense from intangible assets decreased by $245,678, or 100.0%, as compared to the year ended December 31, 2019. At the end of September 2019, our intangible assets were impaired to zero and therefore, no amortization expense was recorded related to intangible assets in the year ended December 31, 2020. |
| ● | For the year ended December 31, 2020, travel and entertainment expense decreased by $347,505, or 66.5%, as compared to the year ended December 31, 2019. The decrease was mainly due to decreased business travel activities and decreased entertainment expenditure resulting from COVID-19. In the year ended December 31, 2020, the spread of COVID-19 has caused public health officials to recommend precautions to mitigate the spread of the virus, such as, cease traveling to non-essential jobs and curtail all unnecessary travel, and stay at home as much as possible. |
| ● | For the year ended December 31, 2020, Directors and Officers Liability Insurance premium increased by $91,605, or 49.7%, as compared to the year ended December 31, 2019. The increase was mainly due to different insurance provider with different premium. |
| ● | For the year ended December 31, 2020, rent and related utilities expenses increased by $1,337, or 1.5%, as compared to the year ended December 31, 2019. |
| ● | Other general and administrative expenses mainly consisted of NASDAQ listing fee, academic sponsorship, and other miscellaneous items. For the year ended December 31, 2020, other general and administrative expenses decreased by $45,282, or 9.9%, as compared to the year ended December 31, 2019, which was mainly due to a decrease in academic sponsorship expenditure of approximately $95,000, offset by an increase in other miscellaneous items of approximately $50,000. |
| ● | In September 2019, we assessed our intangible assets for any impairment and concluded that there were indicators of impairment as of September 30, 2019 and we calculated that the estimated undiscounted cash flows were less than the carrying amount of those intangible assets. We have not been able to realize the financial projections provided by Dr. Zhou at the time of the intangible assets purchase and have decided to impair the intangible assets to zero. Based on our analysis, we recognized an impairment loss of $1,010,011 for the year ended December 31, 2019, which reduced the value of intangible assets purchased to zero. We did not record any impairment charge for the year ended December 31, 2020. |
Loss from Operations
As a result of the foregoing, for the year ended December 31, 2020, loss from operations amounted to $12,454,019, as compared to $19,377,230 for the year ended December 31, 2019, a decrease of $6,923,211, or 35.7%.
Other Income (Expense)
Other income (expense) mainly includes interest expense, change in fair value of warrants liabilities, allocated financing costs, loss from equity method investment, and loss from noncontrolling interest deficit adjustment.
Other expense, net, totaled $225,419 for the year ended December 31, 2020, as compared to other income, net, of $1,307,069 for the year ended December 31, 2019, a decrease of $1,532,488, or 117.2%, which was primarily attributable to a decrease in change in fair value of warrants liabilities of approximately $2,817,000, an increase in interest expense of approximately $86,000, a decrease in other income of approximately $21,000, offset by a decrease in allocated financing expense of approximately $525,000, a decrease in loss from noncontrolling interest deficit adjustment of approximately $862,000, and a decrease in loss from equity method investment of approximately $4,000.
Income Taxes
We did not have any income taxes expense for the years ended December 31, 2020 and 2019 since we incurred losses in these periods.
Net Loss
As a result of the factors described above, our net loss was $12,679,438 for the year ended December 31, 2020, as compared to $18,070,161 for the year ended December 31, 2019, a decrease of $5,390,723 or 29.8%.
Net Loss Attributable to Avalon GloboCare Corp. Common Shareholders
The net loss attributable to Avalon GloboCare Corp. common shareholders was $12,679,438 or $(0.16) per share (basic and diluted) for the year ended December 31, 2020, as compared with $18,070,161, or $(0.24) per share (basic and diluted) for the year ended December 31, 2019, a change of $5,390,723 or 29.8%.
115
Foreign Currency Translation Adjustment
Our reporting currency is the U.S. dollar. The functional currency of our parent company, AHS, Avalon RT 9, Genexosome, Avactis, and Exosome, is the U.S. dollar and the functional currency of Avalon Shanghai and Beijing Genexosome, is the Chinese Renminbi (“RMB”). The financial statements of our subsidiaries whose functional currency is the RMB are translated to U.S. dollars using period end rates of exchange for assets and liabilities, average rate of exchange for revenues, costs, and expenses and cash flows, and at historical exchange rates for equity. Net gains and losses resulting from foreign exchange transactions are included in the results of operations. As a result of foreign currency translations, which are a non-cash adjustment, we reported a foreign currency translation gain of $67,237 and a foreign currency translation loss of $20,887 for the years ended December 31, 2020 and 2019, respectively. This non-cash gain/loss had the effect of decreasing/increasing our reported comprehensive loss.
Comprehensive Loss
As a result of our foreign currency translation adjustment, we had comprehensive loss of $12,612,201 and $18,091,048 for the years ended December 31, 2020 and 2019, respectively.
Liquidity and Capital Resources
The Company has a limited operating history and its continued growth is dependent upon the continuation of providing medical consulting services to its only few clients who are related parties and generating rental revenue from its income-producing real estate property in New Jersey; hence generating revenues, and obtaining additional financing to fund future obligations and pay liabilities arising from normal business operations. In addition, the current cash balance cannot be projected to cover the operating expenses for the next twelve months from the release date of this report. These matters raise substantial doubt about the Company’s ability to continue as a going concern. The ability of the Company to continue as a going concern is dependent on the Company’s ability to raise additional capital, implement its business plan, and generate significant revenues. There are no assurances that the Company will be successful in its efforts to generate significant revenues, maintain sufficient cash balance or report profitable operations or to continue as a going concern. The Company plans on raising capital through the sale of equity to implement its business plan. However, there is no assurance these plans will be realized and that any additional financings will be available to the Company on satisfactory terms and conditions, if any.
The occurrence of an uncontrollable event such as the COVID-19 pandemic is likely to negatively affect the Company’s operations. Efforts to contain the spread of the coronavirus have intensified, including social distancing, travel bans and quarantine, and these are likely to negatively impact our tenants, employees and consultants. These, in turn, will not only impact our operations, financial condition and demand for our medical related consulting services but our overall ability to react timely to mitigate the impact of this event. Given the dynamic nature of these circumstances, the duration of business disruption and reduced traffic, the related financial effect cannot be reasonably estimated at this time but is expected to adversely impact our business for the rest of 2021.
Liquidity is the ability of a company to generate funds to support its current and future operations, satisfy its obligations and otherwise operate on an ongoing basis. At March 31, 2021 and December 31, 2020, we had cash balance of approximately $1,693,000 and $727,000, respectively. These funds are kept in financial institutions located as follows:
| Country: | March 31, 2021 | December 31, 2020 | ||||||||||||||
| United States | $ | 1,527,257 | 90.2 | % | $ | 559,711 | 77.0 | % | ||||||||
| China | 165,283 | 9.8 | % | 166,866 | 23.0 | % | ||||||||||
| Total cash | $ | 1,692,540 | 100.0 | % | $ | 726,577 | 100.0 | % | ||||||||
Under applicable PRC regulations, foreign invested enterprises, or FIEs, in China may pay dividends only out of their accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. In addition, a foreign invested enterprise in China is required to set aside at least 10% of its after-tax profit based on PRC accounting standards each year to its general reserves until the cumulative amount of such reserves reach 50% of its registered capital. These reserves are not distributable as cash dividends.
In addition, a portion of our businesses and assets are denominated in RMB, which is not freely convertible into foreign currencies. All foreign exchange transactions take place either through the People’s Bank of China or other banks authorized to buy and sell foreign currencies at the exchange rates quoted by the People’s Bank of China. Approval of foreign currency payments by the People’s Bank of China or other regulatory institutions requires submitting a payment application form together with suppliers’ invoices, shipping documents and signed contracts. These currency exchange control procedures imposed by the PRC government authorities may restrict the ability of our PRC subsidiary to transfer its net assets to the Parent Company through loans, advances or cash dividends.
The current PRC Enterprise Income Tax (“EIT”) Law and its implementing rules generally provide that a 10% withholding tax applies to China-sourced income derived by non-resident enterprises for PRC enterprise income tax purposes unless the jurisdiction of incorporation of such enterprises’ shareholder has a tax treaty with China that provides for a different withholding arrangement.
116
The following table sets forth a summary of changes in our working capital from December 31, 2020 to March 31, 2021:
| March 31, | December 31, | Changes in | ||||||||||||||
| 2021 | 2020 | Amount | Percentage | |||||||||||||
| Working capital deficit: | ||||||||||||||||
| Total current assets | $ | 2,164,180 | $ | 1,286,337 | $ | 877,843 | 68.2 | % | ||||||||
| Total current liabilities | 3,223,786 | 2,592,393 | 631,393 | 24.4 | % | |||||||||||
| Working capital deficit | $ | (1,059,606 | ) | $ | (1,306,056 | ) | $ | 246,450 | (18.9 | )% | ||||||
Our working capital deficit decreased by $246,450 to $1,059,606 at March 31, 2021 from $1,306,056 at December 31, 2020. The decrease in working capital deficit was primarily attributable to an increase in cash of approximately $966,000, and a decrease in accrued research and development fees of approximately $81,000, offset by a decrease in deferred financing costs of approximately $70,000, an increase in accrued professional fees of approximately $232,000, an increase in accrued liabilities and other payables – related parties of approximately $45,000, an increase in operating lease obligation of approximately $56,000, and an increase in note payable – related party of $390,000.
Because the exchange rate conversion is different for the condensed consolidated balance sheets and the condensed consolidated statements of cash flows, the changes in assets and liabilities reflected on the condensed consolidated statements of cash flows are not necessarily identical with the comparable changes reflected on the condensed consolidated balance sheets.
Cash Flows for the Three Months Ended March 31, 2021 Compared to the Three Months Ended March 31, 2020
The following summarizes the key components of our cash flows for the three months ended March 31, 2021 and 2020:
| Three Months Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| Net cash used in operating activities | $ | (1,515,525 | ) | $ | (1,998,796 | ) | ||
| Net cash used in investing activities | (30,844 | ) | - | |||||
| Net cash provided by financing activities | 2,512,212 | 1,874,877 | ||||||
| Effect of exchange rate on cash | 120 | (5,701 | ) | |||||
| Net increase (decrease) in cash | $ | 965,963 | $ | (129,620 | ) | |||
Net cash flow used in operating activities for the three months ended March 31, 2021 was $1,515,525, which primarily reflected our consolidated net loss of approximately $2,367,000, and the changes in operating assets and liabilities, primarily consisting of an increase in prepaid expenses and other current assets of approximately $41,000, and a decrease in operating lease obligation of approximately $33,000, offset by an increase in accrued liabilities and other payables of approximately $163,000, an increase in accrued liabilities and other payables – related parties of approximately $45,000, and the non-cash items adjustment primarily consisting of depreciation of approximately $79,000, and stock-based compensation and service expense of approximately $574,000.
Net cash flow used in operating activities for the three months ended March 31, 2020 was $1,998,796, which primarily reflected our consolidated net loss of approximately $3,271,000, and the changes in operating assets and liabilities, primarily consisting of an increase in prepaid expenses and other current assets of approximately $97,000, offset by a decrease in accounts receivable – related party of approximately $86,000, and the non-cash items adjustment primarily consisting of depreciation of approximately $77,000, and stock-based compensation and service expense of approximately $1,189,000.
We expect our cash used in operating activities to increase due to the following:
| ● | the development and commercialization of new products; |
| ● | an increase in professional staff and services; and |
| ● | an increase in public relations and/or sales promotions for existing and/or new brands as we expand within existing markets or enter new markets. |
Net cash flow used in investing activities was $30,844 for the three months ended March 31, 2021. During the three months ended March 31, 2021, we made additional investment in equity method investment of approximately $31,000. There were no investing activities during the three months ended March 31, 2020.
117
Net cash flow provided by financing activities was $2,512,212 for the three months ended March 31, 2021 as compared to $1,874,877 for the three months ended March 31, 2020. During the three months ended March 31, 2021, we received proceeds from related party borrowings of approximately $105,000 and net proceeds from equity offering of approximately $2,407,000 (net of cash paid for commission of approximately $74,000). During the three months ended March 31, 2020, we received proceeds from related party borrowings of $300,000 and net proceeds from equity offering of approximately $1,575,000 (net of cash paid for commission of approximately $49,000).
Cash Flows for the Year Ended December 31, 2020 Compared to the Year Ended December 31, 2019
The following summarizes the key components of our cash flows for the years ended December 31, 2020 and 2019:
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Net cash used in operating activities | $ | (7,546,100 | ) | $ | (7,079,871 | ) | ||
| Net cash used in investing activities | (169,185 | ) | (552,967 | ) | ||||
| Net cash provided by financing activities | 7,664,281 | 6,154,910 | ||||||
| Effect of exchange rate on cash | 12,690 | (9,468 | ) | |||||
| Net decrease in cash | $ | (38,314 | ) | $ | (1,487,396 | ) | ||
Net cash flow used in operating activities for the year ended December 31, 2020 was $7,546,100, which primarily reflected our consolidated net loss of approximately $12,679,000, and the changes in operating assets and liabilities, primarily consisting of an increase in prepaid expenses and other current assets of approximately $207,000, a decrease in accrued liabilities and other payables of approximately $837,000, offset by a decrease in accounts receivable – related party of approximately $217,000, an increase in accrued liabilities and other payables – related parties of approximately $119,000, and the non-cash items adjustment primarily consisting of depreciation and amortization of approximately $315,000, and stock-based compensation and service expense of approximately $5,494,000.
Net cash flow used in operating activities for the year ended December 31, 2019 was $7,079,871, which primarily reflected our consolidated net loss of approximately $18,070,000, the non-cash item adjustment consisting of change in warrants derivative liabilities of approximately $2,817,000, and the changes in operating assets and liabilities, primarily consisting of an increase in accounts receivable – related party of approximately $217,000, offset by a decrease in prepaid expenses and other current assets of approximately $480,000, and an increase in accrued liabilities and other payables of approximately $1,230,000, and the add-back of non-cash items mainly consisting of depreciation and amortization of approximately $507,000, stock-based compensation and service expense of approximately $9,209,000, allocated financing costs of approximately $525,000, impairment loss of approximately $1,010,000, and loss from noncontrolling interest deficit adjustment of approximately $862,000.
Net cash flow used in investing activities was $169,185 for the year ended December 31, 2020 as compared to $552,967 for the year ended December 31, 2019. During the year ended December 31, 2020, we made payment for improvement of commercial real estate of approximately $111,000 and made additional investment in equity method investment of approximately $58,000.
During the year ended December 31, 2019, we made payment for purchase of property and equipment of approximately $377,000, made payment for improvement of commercial real estate of approximately $16,000, and made payment for equity method investment of approximately $159,000.
Net cash flow provided by financing activities was $7,664,281 for the year ended December 31, 2020 as compared to $6,154,910 for the year ended December 31, 2019. During the year ended December 31, 2020, we received proceeds from related party borrowings of $600,000 and net proceeds from equity offering of approximately $7,264,000 (net of cash paid for commission and offering costs of approximately $540,000), offset by repayments made for note payable – related party of $200,000.
During the year ended December 31, 2019, we received proceeds from borrowings from a related party of $3,600,000, and net proceeds from equity offering of approximately $5,365,000 (net of offering costs of approximately $909,000), offset by repayments made to a related party for borrowings of $410,000, repayments for loan payable of $1,000,000, and payment made for repurchase of warrants of 1,400,000.
118
Our capital requirements for the next twelve months primarily relate to working capital requirements, including salaries, fees related to third parties’ professional services, reduction of accrued liabilities, mergers, acquisitions and the development of business opportunities. These uses of cash will depend on numerous factors including our sales and other revenues, and our ability to control costs. All funds received have been expended in the furtherance of growing the business. The following trends are reasonably likely to result in a material decrease in our liquidity over the near to long term:
| ● | an increase in working capital requirements to finance our current business, including ongoing research and development programs, clinical studies, as well as commercial strategies; | |
| ● | the use of capital for mergers, acquisitions and the development of business opportunities; | |
| ● | addition of administrative personnel as the business grows; and |
| ● | the cost of being a public company. |
In the third quarter of 2019, we had secured a $20 million credit facility (Line of Credit) provided by our Chairman, Wenzhao Lu. The unsecured credit facility bears interest at a rate of 5% and provides for maturity on drawn loans 36 months after funding. The note is not convertible to equity. As of March 31, 2021, the total principal amount outstanding under the Credit Line was approximately $3.3 million and we have approximately $16.7 million remaining available under the Line Credit.
On December 13, 2019, we entered into an Open Market Sale AgreementSM (the “Sales Agreement”) with Jefferies LLC, as sales agent (“Jefferies”), pursuant to which we may offer and sell, from time to time, through Jefferies, shares of our common stock, par value $0.0001 per share, having an aggregate offering price of up to $20.0 million. On April 6, 2020, the date on which we filed our Annual Report on Form 10-K for the fiscal year ended December 31, 2019, our registration statement became subject to the offering limits set forth in General Instruction I.B.6 of Form S-3. As of April 6, 2020, the aggregate market value of our outstanding common stock held by non-affiliates, or public float, was $39,564,237, based on 23,691,160 shares of our outstanding common stock that were held by non-affiliates on such date and a price of $1.67 per share, which was the price at which our common stock was last sold on The Nasdaq Capital Market on February 19, 2020 (a date within 60 days of the date hereof), calculated in accordance with General Instruction I.B.6 of Form S-3. We have not offered any securities pursuant to General Instruction I.B.6 of Form S-3 in the 12 calendar months preceding the date of this prospectus supplement. We filed a prospectus supplement to amend and supplement the information in our prospectus and original prospectus supplement based on the amount of securities that we are eligible to sell under General Instruction I.B.6 of Form S-3. After giving effect to the $13,000,000 offering limit imposed by General Instruction I.B.6 of Form S-3, we may offer and sell additional shares of our common stock having an aggregate offering price of up to $13,000,000 from time to time through Jefferies acting as our sales agent in accordance with the terms of the sales agreement. As of March 31, 2021, we sold a total of 5,900,275 shares of our common stock through Jefferies with an aggregate offering price of $9,559,240 and we have approximately $5.4 million offering price remaining available under the Sales Agreement.
We estimate that based on current plans and assumptions, our available cash will be insufficient to satisfy our cash requirements under our present operating expectations, and we expect to need to use our Credit Line and/or sales of equity through our Sales Agreement.
Avalon and SenlangBio currently expect that SenlangBio’s cash reserves, based on cash balances at March 31, 2021, together with the proceeds from the Equity Financing, will fund SenlangBio’s operations for 18 months following the closing of the Acquisition, based on current plans and assumptions.
Other than funds received from the sale of our equity and advances from our related party, and cash resource generating from our operations, we presently have no other significant alternative source of working capital. We have used these funds to fund our operating expenses, pay our obligations and grow our company. We will need to raise significant additional capital to fund our operations and to provide working capital for our ongoing operations and obligations. Therefore, our future operation is dependent on our ability to secure additional financing. Financing transactions may include the issuance of equity or debt securities, obtaining credit facilities, or other financing mechanisms. However, the trading price of our common stock and a downturn in the U.S. equity and debt markets could make it more difficult to obtain financing through the issuance of equity or debt securities. Even if we are able to raise the funds required, it is possible that we could incur unexpected costs and expenses or experience unexpected cash requirements that would force us to seek alternative financing. Furthermore, if we issue additional equity or debt securities, stockholders may experience additional dilution or the new equity securities may have rights, preferences or privileges senior to those of existing holders of our common stock. The inability to obtain additional capital may restrict our ability to grow and may reduce our ability to continue to conduct business operations. If we are unable to obtain additional financing, we will be required to cease our operations. To date, we have not considered this alternative, nor do we view it as a likely occurrence.
119
Contractual Obligations and Off-Balance Sheet Arrangements
Contractual Obligations
We have certain fixed contractual obligations and commitments that include future estimated payments. Changes in our business needs, cancellation provisions, and other factors may result in actual payments differing from the estimates. We cannot provide certainty regarding the timing and amounts of payments. We have presented below a summary of the most significant assumptions used in our determination of amounts presented in the tables, in order to assist in the review of this information within the context of our consolidated financial position, results of operations, and cash flows. The following tables summarize our contractual obligations as of March 31, 2021, and the effect these obligations are expected to have on our liquidity and cash flows in future periods.
| Payments Due by Period | ||||||||||||||||||||
| Contractual obligations: | Total | Less than 1 year | 1-3 years | 3-5 years | 5+ years | |||||||||||||||
| Operating lease commitment | $ | 273,108 | $ | 148,752 | $ | 124,356 | $ | - | $ | - | ||||||||||
| Acquisition consideration | 100,000 | 100,000 | - | - | - | |||||||||||||||
| Borrowings from related party (principal) | 3,695,249 | 390,000 | 3,305,249 | - | - | |||||||||||||||
| Accrued interest – related party | 213,105 | 213,105 | - | - | - | |||||||||||||||
| Epicon equity investment obligation | 808,827 | 269,609 | 539,218 | - | - | |||||||||||||||
| AVAR joint venture commitment | 10,763,044 | 763,044 | 5,000,000 | 5,000,000 | - | |||||||||||||||
| Total | $ | 15,853,333 | $ | 1,884,510 | $ | 8,968,823 | $ | 5,000,000 | $ | - | ||||||||||
Off-balance Sheet Arrangements
We presently do not have off-balance sheet arrangements.
Foreign Currency Exchange Rate Risk
A portion of our operations are in China. Thus, a portion of our revenues and operating results may be impacted by exchange rate fluctuations between RMB and US dollars. For the three months ended March 31, 2021 and 2020, we had an unrealized foreign currency translation loss of approximately $3,000 and $22,000, respectively, because of changes in the exchange rate.
Inflation
The effect of inflation on our revenue and operating results was not significant.
120
MANAGEMENT AFTER THE ACQUISITION
Executive Officers and Directors of Avalon After the Acquisition
It is currently expected that Dr. Jianqiang Li, scientific founder and CSO of SenlangBio, will join the board of Avalon, and Dr. Li will also be appointed as Chief Technology Officer of Avalon. Meng Li, Avalon’s Chief Operating Officer and a director, will resign from her position on the board of Avalon. The Avalon board and management will otherwise remain the same aside from Dr. Li, with Wenzhao Lu (Chairman), David Jin, MD, PhD, Steven A. Sanders, Yancen Lu, Wilbert J. Tauzin II, Willliam B. Stilley, III, Tevi Troy and Yue “Charles” Li continuing to serve on the board and Dr. Jin continuing to serve as President and Chief Executive Officer, Ms. Li as Chief Operating Officer and Luisa Ingargiola as Chief Financial Officer. The reconstituted board is expected to satisfy the requisite independence requirements for the Avalon board of directors, as well as the sophistication and independence requirements for the required committees pursuant to Nasdaq listing requirements.
The following table lists the names and positions of the individuals currently identified to serve as executive officers and directors of Avalon upon the completion of the Acquisition:
Executive Officers
| Name | Age | Position | ||
| David Jin, MD, PHD | 53 | Chief Executive Officer and President | ||
| Dr. Jianqiang Li | 48 | Chief Technology Officer | ||
| Meng Li | 43 | Chief Operating Officer and Secretary | ||
| Luisa Ingargiola | 53 | Chief Financial Officer |
Directors
| Name | Age | Position | ||
| Wenzhao “Daniel” Lu | 63 | Chairman of the Board of Directors | ||
| David Jin, MD, PhD | 53 | Chief Executive Officer, President and Director | ||
| Dr. Jianqiang Li | 48 | Chief Technology Officer and Director | ||
| Steven A. Sanders | 75 | Director | ||
| Yancen Lu | 46 | Director | ||
| Wilbert J. Tauzin II | 76 | Director | ||
| William B. Stilley, III | 53 | Director | ||
| Tevi Troy | 53 | Director | ||
| Yue “Charles” Li | 47 | Director |
121
Executive Officers
David Jin, Chief Executive Officer, President and Director
Dr. David Jin, MD, PhD, is Avalon’s Chief Executive Officer, President and a member of the Board of Directors. From 2009 to 2017, Dr. Jin has served as the Chief Medical Officer of BioTime, Inc. (NYSE American: BTX), a clinical stage regenerative medicine company with a focus on pluripotent stem cell technology. Dr. Jin also acts as a senior translational clinician-scientist at the Howard Hughes Medical Institute and the Ansary Stem Cell Center at Weill Cornell Medical College of Cornell University. Prior to his current endeavors, Dr. Jin was Chief Consultant/Advisor for various biotech/pharmaceutical companies regarding hematology, oncology, immunotherapy and stem cell-based technology development. Dr. Jin has been Principle Investigator in more than 15 pre-clinical and clinical trials, as well as author/co-author of over 80 peer-reviewed scientific abstracts, articles, reviews, and book chapters. Dr. Jin studied medicine at SUNY Downstate College of Medicine in Brooklyn, New York. He received his clinical training and subsequent faculty tenure at the New York-Presbyterian Hospital (the teaching hospital for both Cornell and Columbia Universities) in the areas of internal medicine, hematology, and clinical oncology. Dr. Jin was honored as Top Chief Medical Officer by ExecRank in 2012, as well as recognized by Leading Physicians of the World in 2015. Dr. Jin is qualified to serve as a director because of his role with Avalon and his extensive operational knowledge of, and executive level management experience in, the healthcare industry.
Jianqiang Li, Ph.D., Chief Technology Officer and Director
Jianqiang Li has over 20 years of academic and industry experience in immunology and cell therapy. Dr. Li has published 13 papers in top-ranked journals, including Nature Medicine, Blood, and the Journal of Immunology, with a cumulative impact index over 60. Dr. Li is an invited speaker at ASH, ASBMT, ICBS, ECI and Gamma-Delta T Cell International Conference. Dr. Li independently set up an efficient CAR-T production system, achieved major breakthroughs in the development and clinical application of allogeneic CAR γδT-cell therapy, and made important contributions to the antigen presentation and activation mechanism of Vγ9Vδ2 T-cells. He has successfully developed CAR-γδ T-cell products derived from human cord blood. Dr. Li has a Master of Immunology from the Institute of Basic Medicine, Peking Union Medical College (2003-2006), a Doctor of Immunology from the University of Würzburg (2006-2009), was a Postdoctoral Fellow at the City of Hope National Medical Center (2010) and was a Senior Scientist in the R&D Department at Fred Hutchinson Cancer Research Center (2011-2016), before founding SenlangBio.
Meng Li, Chief Operating Officer and Secretary
Ms. Meng Li is Avalon’s Chief Operating Officer and Secretary and a member of the Board of Directors. Previously, Ms. Li served on the Board of Directors from October 2017 through July 2018 and was re-appointed in February 2019. Ms. Li has over 15 years of executive experience in international marketing, branding, communications, and media investment consultancy. Ms. Li served as Managing Director at Maxus/GroupM (a WPP Group company) where she was responsible for business P&L and corporate management from 2006 to 2015. Prior to joining Maxus/Group M, Ms. Li worked for Zenith Media (a Publicis Group company) from 2000 to 2006 as Senior Manager. Ms. Li received a Bachelor of Arts in International Economic Law from Dalian Maritime University in China. Ms. Li is qualified to serve as a director because of her role with Avalon and her executive level management experience.
Luisa Ingargiola, Chief Financial Officer
Luisa Ingargiola is the Company’s Chief Financial Officer. Ms Ingargiola has significant experience serving as Chief Financial Officer or Audit Chair for multiple NASDAQ and NYSE companies. She currently serves as Director and Audit Chair for several public companies including ElectraMeccanica (NASDAQ:SOLO), AgEagle (NYSE:UAVS), Siyata Mobile (NASDAQ:SYTA) and Progress Acquisition Corporation (NASDAQ:PGRWU). From 2007 through 2016, Ms. Ingargiola served as the Chief Financial Officer and then Director at MagneGas Corporation (Nasdaq: MNGA). Prior to 2007, Ms. Ingargiola held various roles as Budget Director and Investment Analyst in several private companies. Ms. Ingargiola graduated in 1989 from Boston University with a Bachelor’s degree in Business Administration and a concentration in Finance. In 1996, she received her MBA in Health Administration from the University of South Florida. Ms. Ingargiola is qualified to serve as a Chief Financial Officer because of her extensive knowledge corporate governance, regulatory requirements, executive leadership and knowledge of, and experience in, financing and M&A transactions.
122
Directors
Information concerning Dr. Jin and Dr. Li is provided under “Management After the Acquisition— Executive Officers and Directors of the Post-Acquisition company After the Acquisition—Executive Officers” above.
Wenzhao “Daniel” Lu, Chairman of the Board of Directors
Mr. Wenzhao Lu is Avalon’s Chairman of the Board. He is a seasoned healthcare entrepreneur with extensive operational knowledge and experience in China. He has been serving as Chairman of the Board for the Daopei Medical Group, or DPMG, since 2010. Under his leadership, DPMG has recently expanded its clinical network involving a state-of-the-art stem cell bank at Wuhan Biolake, three top-ranked private hospitals (located in Beijing, Shanghai, and Hebei), specialty hematology laboratories, as well as a hematology research institute, with more than 100 partnering and collaborating hospitals in China. DPMG was founded by Professor Daopei Lu, a renowned hematologist pioneering in hematopoietic stem cell transplant and member of the Academy of Engineering in China. Mr. Wenzhao Lu received a Bachelor of Arts from Temple University Tyler School of Arts in 1988 and subsequently worked as senior Art Director at Ogilvy & Mather Advertising Company. Prior to joining DPMG, Mr. Lu served as Chief Operating Officer for BioTime Asia Limited, which is a subsidiary of BioTime, Inc. (NYSE American: BTX) in 2009. Mr. Lu is qualified to serve as a director because of his extensive operational knowledge of, and executive level management experience in, the healthcare industry.
Steven A. Sanders, Director
Steven A. Sanders is a member of the Board of Directors. Since January 2017, Mr. Sanders has been Of Counsel to the law firm of Ortoli Rosenstadt LLP. From July 2007 until January 2017, Mr. Sanders was a Senior Partner of Ortoli Rosenstadt LLP. From January 1, 2004 until June 30, 2007, he was Of Counsel to the law firm of Rubin, Bailin, Ortoli, LLP. From January 1, 2001 to December 31, 2003, he was Counsel to the law firm of Spitzer & Feldman PC. Mr. Sanders also serves as a Director of Helijet International, Inc. and Electrameccanica Vehicles Corp. (NASDAQ:SOLO). Additionally, he has been a director at the American Academy of Dramatic Arts since October 2013 and has been a director of the Bay Street Theater since February 2015. Mr. Sanders received his JD from Cornell University and his BBA from The City College of New York. Mr. Sanders is qualified to serve as a director because of his corporate, securities and international law experience, including working with companies in the life sciences industry.
Yancen Lu, Director
Yancen Lu is a member of the Board of Directors. Mr. Lu has more than 20 years of experience in investment banking and equity investment management. He is the Founder and CEO of PagodaTree Partners, a healthcare PE fund. Before this, Mr. Lu was the Managing Director of FountainVest Partners. In addition to his professionalism in securities, investment and capital management, Mr. Lu has a special focus and comprehensive understanding of the global medical and healthcare industry. He served as Director of leading healthcare corporations including Sino Hospital Investment Corporation (Hong Kong), Chang’an Hospital (the largest private hospital in Northwest China), and DIH Medical Technologies. Mr. Lu received Bachelor’s and Master’s degrees in Engineering Economics from Tianjin University. Mr. Lu is qualified to serve as a director because of his extensive operational knowledge of, and executive level management experience in, the healthcare industry.
Wilbert J. Tauzin II, Director
Wilbert J. Tauzin II is a member of the Board of Directors. From December 2010 until March 1, 2014, Congressman Tauzin served as Special Legislative Counsel to Alston & Bird LLP. From December 2004 to June 2010, Congressman Tauzin was President and Chief Executive Officer of the Pharmaceutical Research and Manufacturers of America, a trade group that serves as one of the pharmaceutical industry’s top lobbying groups. He served 12.5 terms in the U.S. House of Representatives, representing Louisiana’s 3rd Congressional District. From January 2001 through February 2004, Congressman Tauzin served as Chairman of the House Committee on Energy and Commerce. He also served as a senior member of the House Resources Committee and Deputy Majority Whip. Prior to serving as a member of Congress, Congressman Tauzin was a member of the Louisiana State Legislature, where he served as Chairman of the House Natural Resources Committee and Chief Administration Floor Leader. He currently serves as lead independent director of LHC Group, a publicly traded provider of quality home health care. Congressman Tauzin received a Bachelor of Arts Degree from Nicholls State University and a Juris Doctor degree from Louisiana State University. Congressman Tauzin is qualified to serve as a director because of his extensive knowledge of the pharmaceutical industry and his experience as a director of several publicly-traded and privately-held companies.
123
William B. Stilley, III, Director
William B. Stilley is a member of the Board of Directors. Mr. Stilley has been the chief executive officer and member of the board of directors of Adial Pharmaceuticals, Inc. since December 2010. From August 2008 until December 2010, he was the vice president, business development and strategic projects at Clinical Data, Inc. (NASDQ: CLDA). From February 2002, Mr. Stilley was the COO and CFO of Adenosine Therapeutics, LLC until certain assets of Adenosine Therapeutics were acquired by Clinical Data, Inc. in August 2008. Mr. Stilley has advised both public and private companies on financing and M&A transactions, has been the interim CFO of a public company, the interim Chief Business Officer and then Advisor for Diffusion Pharmaceuticals from September 2015 through March 2018, and the COO and CFO of a number of private companies. Before entering the business community, Mr. Stilley served as Captain in the U.S. Marine Corps. Mr. Stilley has an MBA with honors from the Darden School of Business and a B.S. in Commerce/Marketing from the McIntire School of Commerce at the University of Virginia. He currently serves on the Advisory Board of Virginia BIO, the statewide biotechnology organization. Mr. Stilley is qualified to serve as a director because of his extensive knowledge of the biotechnology industry, significant executive leadership and operational experience, and knowledge of, and experience in, financing and M&A transactions.
Tevi Troy, Director
Tevi Troy is a member of the Board of Directors and a former Deputy Secretary of the U.S. Department of Health and Human Services. Dr. Troy has previously been the founder and CEO of the American Health Policy Institute and a Senior Fellow at Hudson Institute, where he remains an Adjunct Fellow. On August 3, 2007, Dr. Troy was unanimously confirmed by the U.S. Senate as the Deputy Secretary of HHS. As Deputy Secretary, Dr. Troy was the chief operating officer of the largest civilian department in the federal government, with a budget of $716 billion and over 67,000 employees. Dr. Troy has extensive White House experience, having served in several high-level positions over a five-year period, culminating in his service as Deputy Assistant and then Acting Assistant to the President for Domestic Policy. Dr. Troy has held high-level positions on Capitol Hill as well. From 1998 to 2000, Dr. Troy served as the Policy Director for Senator John Ashcroft. From 1996 to 1998, Dr. Troy was Senior Domestic Policy Adviser and later Domestic Policy Director for the House Policy Committee, chaired by Christopher Cox. In addition to his senior level government work and health care expertise, Dr. Troy is also a best-selling presidential historian and the author of five books, including, most recently, "Fight House: Rivalries in the White House from Truman to Trump," which the Wall Street Journal listed as one of the top political books of 2020. Dr. Troy’s many other affiliations include: contributing editor for Washingtonian magazine; member of the publication committee of National Affairs; member of the Board of Fellows of the Jewish Policy Center; a Senior Fellow at the Potomac Institute; and a member of the Bipartisan Commission on Biodefense. Dr. Troy has a B.S. in Industrial and Labor Relations from Cornell University and an M.A and Ph.D. in American Civilization from the University of Texas at Austin. Dr. Troy is qualified to serve as a director because of his extensive knowledge of the healthcare industry and his significant leadership experience.
Yue “Charles” Li
Yue “Charles” Li is a member of the Board of Directors. Mr. Li has about 20 years of experience in M&A and capital markets in China and the U.S. Mr. Li currently is a Managing Director at PagodaTree Partners, a private equity company with a focus on healthcare in Beijing. Prior to PagodaTree, he was a senior executive at a major conglomerate in China where he successfully closed $2 billion M&A transactions in healthcare and insurance areas. Previously, Mr. Li spent 8 years in Deloitte, as a director of financial advisory services in Beijing and capital markets in New York. His key clients included Merrill Lynch, Blackrock, KKR etc. In his early career, Mr. Li served for top tier financial institutions such as Credit Suisse and Fannie Mae, responsible for asset allocation strategy and risk management for multibillion USD portfolios. Mr. Li received Master’s degree from the Olin School of Business at Washington University in 2000 and a Bachelor of Engineering from Tianjin University in 1996. He is a CFA charter holder. Mr. Li is qualified to serve as a director because of his extensive investment and executive level management experience.
Director Independence
Upon the consummation of the Acquisition, the board of directors of Avalon is expected to determine that each the directors on the board of directors other than Dr. Li, Wenzhao Lu, David Jin, MD, PhD and Wilbert J. Tauzin II, will qualify as independent directors, as defined under the listing rules of The Nasdaq Stock Market LLC and the board of directors will consist of a majority of “independent directors,” as defined under the rules of the SEC and Nasdaq listing rules relating to director independence requirements. In addition, Avalon will be subject to the rules of the SEC and Nasdaq relating to the membership, qualifications, and operations of the audit committee, as discussed below.
Board Procedures and Committees
No material changes are currently contemplated to the procedures of the board of directors or any committees established thereby in light of the Acquisition.
124
DESCRIPTION OF AVALON’S SECURITIES
We have authorized capital stock consisting of 490,000,000 shares of common stock, par value $0.0001 per share, and 10,000,000 shares of preferred stock, par value $0.0001 per share. As of June 30, 2021, we had 85,080,919 shares of common stock issued and outstanding, and no shares of preferred stock issued and outstanding.
Common Stock
All outstanding shares of common stock are of the same class and have equal rights and attributes. The holders of common stock are entitled to one vote per share on all matters submitted to a vote of stockholders of the company. All stockholders are entitled to share equally in dividends, if any, as may be declared from time to time by the Board of Directors out of funds legally available. In the event of liquidation, the holders of common stock are entitled to share ratably in all assets remaining after payment of all liabilities. The stockholders do not have cumulative or preemptive rights.
Preferred Stock
Our Certificate of Incorporation authorizes the issuance of up to 10,000,000 shares of preferred stock with designations, rights and preferences determined from time to time by our Board of Directors. Accordingly, our Board of Directors is empowered, without stockholder approval, to issue preferred stock with dividend, liquidation, conversion, voting, or other rights which could adversely affect the voting power or other rights of the holders of the common stock. In the event of issuance, the preferred stock could be utilized, under certain circumstances, as a method of discouraging, delaying or preventing a change in control of our company, which is sometimes referred to in corporate parlance as a “poison pill”.
Stockholder Action by Written Consent
Any action required or permitted to be taken at any annual or special meetings of the stockholders of the company may be taken without a meeting, without prior notice and without a vote, by a consent or consents in writing, setting forth the action so taken, (a) signed by stockholders of the company holding not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all the shares of the company entitled to vote thereon were present and voted and (b) delivered to the company in accordance with Section 228 of the DGCL.
Anti-Takeover Effects of Provisions of our Certificate of Incorporation, our Bylaws and Delaware Law
Some provisions of Delaware law, our certificate of incorporation and our bylaws contain provisions that could make the following transactions more difficult: acquisition of us by means of a tender offer; acquisition of us by means of a proxy contest or otherwise; or removal of our incumbent officers and directors. It is possible that these provisions could make it more difficult to accomplish or could deter transactions that stockholders may otherwise consider to be in their best interest or in our best interests, including transactions that might result in a premium over the price of our common stock.
These provisions, summarized below, are expected to discourage coercive takeover practices and inadequate takeover bids. These provisions are also designed to encourage persons seeking to acquire control of us to first negotiate with our board of directors. We believe that the benefits of increased protection of our potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure us outweigh the disadvantages of discouraging these proposals because negotiation of these proposals could result in an improvement of their terms.
Delaware Anti-Takeover Statute
We are subject to Section 203 of the Delaware General Corporation Law, which regulates corporate takeovers. The existence of this provision may have an anti-takeover effect with respect to transactions not approved in advance by the board of directors, such as discouraging takeover attempts that might result in a premium over the price of our common stock.
Undesignated Preferred Stock
The ability to authorize undesignated preferred stock makes it possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change control. These and other provisions may have the effect of deterring hostile takeovers or delaying changes in control or management.
Transfer Agent
The stock transfer agent for our securities is Vstock Transfer, LLC, 18 Lafayette Place, Woodmere, NY 11598, (212) 828-8436.
125
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
Avalon
The following table sets forth information regarding the beneficial ownership of Avalon Common Stock as of June 30, 2021 by:
| ● | each person known by us to be the beneficial owner of more than 5% of outstanding shares of Avalon Common Stock; |
| ● | each of Avalon’s named executive officers; |
| ● | each of Avalon’s directors; and |
| ● | all of Avalon’s directors and current executive officers as a group. |
Except as may be indicated in the footnotes to this table and pursuant to applicable community property laws, each person named in the table has sole voting and dispositive power with respect to the shares of common stock set forth opposite that person’s name.
| Name of Beneficial Owner (1) | Common Stock Beneficially Owned | Percentage of Common Stock (2) | ||||||
| Wenzhao Lu* (3) | 30,045,161 | 32.7 | % | |||||
| David Jin, MD, PhD* (4) | 16,000,000 | 17.4 | % | |||||
| Meng Li* (5) | 5,600,000 | 6.1 | % | |||||
| Luisa Ingargiola* (6) | 2,400,000 | 2.6 | % | |||||
| Yancen Lu* (7) | 5,390,000 | 5.9 | % | |||||
| Steven A. Sanders* (8) | 190,000 | ** | ||||||
| Wilbert J. Tauzin II* (9) | 710,000 | ** | ||||||
| William B. Stilley III* (10) | 190,000 | ** | ||||||
| Tevi Troy* (11) | 190,000 | ** | ||||||
| Yue (Charles) Li* (12) | 150,000 | ** | ||||||
| All officers and directors as a group (10 persons) | 60,865,161 | 66.3 | % | |||||
| * | Officer and/or director of our company. |
| ** | Less than 1.0%. |
| (1) | Except as otherwise indicated, the address of each beneficial owner is c/o Avalon GloboCare Corp., 4400 Route 9 South, Suite 3100, Freehold, New Jersey 07728. |
| (2) | Applicable percentage ownership is based on 85,080,919 shares of common stock outstanding as of June 30, 2021, together with securities exercisable or convertible into shares of common stock within 60 days of June 30, 2021 for each stockholder. Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission and generally includes voting or investment power with respect to securities. Shares of common stock that are currently exercisable or exercisable within 60 days of June 30, 2021 are deemed to be beneficially owned by the person holding such securities for the purpose of computing the percentage of ownership of such person, but are not treated as outstanding for the purpose of computing the percentage ownership of any other person. |
| (3) | Wenzhao Lu holds (i) 28,545,161 shares of common stock and (ii) 1,500,000 vested options to acquire 1,500,000 shares of common stock of our company. |
| (4) | David Jin holds (i) 15,450,000 shares of common stock and (ii) 550,000 vested options to acquire 550,000 shares of common stock of our company. |
| (5) | Meng Li holds (i) 5,150,000 shares of common stock and (ii) 450,000 vested options to acquire 450,000 shares of common stock of our company. |
| (6) | Represents 2,400,000 vested options to acquire 2,400,000 shares of common stock of our company. |
| (7) | Yancen Lu holds (i) 5,000,000 shares of common stock and (ii) 390,000 vested options to acquire 390,000 shares of common stock of our company. |
| (8) | Represents stock option to acquire 190,000 shares of common stock of our company. |
| (9) | Represents stock option to acquire 710,000 shares of common stock of our company. |
| (10) | Represents stock option to acquire 190,000 shares of common stock of our company. |
| (11) | Represents stock option to acquire 190,000 shares of common stock of our company. |
| (12) | Represents stock option to acquire 150,000 shares of common stock of our company. |
126
OTHER MATTERS
The Board of Directors knows of no other business which will be presented at the Annual Meeting. If any other matters properly come before the meeting, the persons named in the enclosed Proxy, or their substitutes, will vote the shares represented thereby in accordance with their judgment on such matters.
Annual Reports on Form 10-K
Additional copies of Avalon’s Annual Report on Form 10-K for the fiscal year ended December 31, 2020 may be obtained without charge by writing to the Chief Financial Officer, Avalon GloboCare Corp., 4400 Route 9 South, Suite 3100, Freehold, New Jersey 07728. Avalon’s Annual Report on Form 10-K can also be found on Avalon’s website: www.avalon-globocare.com.
Stockholders Proposals for the 2022 Annual Meeting.
Stockholder proposals intended to be presented at the Company’s 2022 annual meeting must be received by the Company no later than [●], 2022 (pursuant to Rule 14a-8 of the Exchange Act, 120 days before the anniversary of the prior year’s mailing date) to be eligible for inclusion in the Company’s proxy statement and form of proxy for next year’s meeting. Proposals should be addressed to Avalon GloboCare Corp., Attn. Chief Financial Officer, 4400 Route 9 South, Suite 3100, Freehold, New Jersey 07728.
For any proposal that is not submitted for inclusion in next year’s proxy statement (as described in the preceding paragraph), but is instead sought to be presented directly at the 2022 annual meeting, the federal securities laws require stockholders to give advance notice of such proposals. The required notice must (pursuant to Rule 14a-4 of the Exchange Act) be given no less than 45 days in advance of the one year anniversary date of the date on which the Company first sent its proxy materials for the immediately preceding annual meeting. Accordingly, with respect to the Company’s 2022 annual meeting of stockholders, notice must be provided to Avalon GloboCare Corp., Attn. Chief Financial Officer, 4400 Route 9 South, Suite 3100, Freehold, New Jersey 07728 no later than [●], 2022. If a stockholder fails to provide timely notice of a proposal to be presented at the 2020 annual meeting, the chair of the meeting will declare it out of order and disregard any such matter.
STOCKHOLDER COMMUNICATION WITH THE BOARD
Our Board believes that it is important for current and potential stockholders and other interested parties to have a process to send communications to the Board. Accordingly, stockholders and other interested parties desiring to send a communication to the Board, or to a specific director, may do so by sending a letter to our executive offices at Avalon GloboCare Corp., Attn. Secretary, 4400 Route 9 South, Suite 3100, Freehold, New Jersey 07728. The mailing envelope must contain a clear notation indicating that the enclosed letter is a “stockholder-board communication” or “stockholder-director communication.” All such letters must identify the author as either a stockholder or non-stockholder and clearly state whether the intended recipients of the letter are all members of the Board or certain specified individual directors. The Secretary will open such communications, make copies, and then circulate them to the appropriate director or directors.
AVAILABILITY OF ANNUAL REPORT
The Company’s Annual Report includes its Annual Report on Form 10-K for the year ended December 31, 2020 (without exhibits) as filed with the SEC. The Company will furnish without charge upon written request a copy of its Annual Report on Form 10-K. The Annual Report on Form 10-K includes a list of all exhibits thereto. The Company will furnish copies of such exhibits upon written request and payment of its reasonable expenses in furnishing such exhibits. Each such request must include a good faith representation that, as of the record date for the annual meeting, the person making such request was a beneficial owner of the Company’s common stock entitled to vote at the annual meeting. Such written request should be directed to the Company’s Secretary, 4400 Route 9 South, Suite 3100, Freehold, New Jersey 07728, (732) 780-4400.
127
Householding—Delivery of Documents to Stockholders
Pursuant to the rules of the SEC, Avalon and servicers that it employs to deliver communications to its stockholders are permitted to deliver to two or more stockholders sharing the same address a single copy of the proxy statement. This process, which is commonly referred to as “householding,” potentially means extra convenience for stockholders and cost savings for companies. As permitted by the Exchange Act, only one copy of this proxy statement will be delivered to multiple Avalon stockholders sharing an address unless contrary instructions have been received by from the impacted stockholders. Once you have received notice from Avalon (if you are an Avalon stockholder of record) or from your broker (if you are a beneficial owner of Avalon Common Stock) that Avalon or they will be “householding” communications to your address, “householding” will continue until you are notified otherwise or until you revoke your consent. If, at any time, you no longer wish to participate in “householding” and would prefer to receive separate copies of Avalon’s annual disclosure documents and this proxy statement or if you currently receive multiple copies and would like to request “householding” of these communications, please notify your broker or Avalon. Direct your request to Avalon by calling or writing Avalon at its principal executive offices at (732) 780-4400 and 4400 Route 9 South, Suite 3100, Freehold, New Jersey 07728.
WHERE YOU CAN FIND MORE INFORMATION
Avalon files reports, proxy statements and other information with the SEC as required by the Exchange Act. The SEC maintains a website that contains reports, proxy statements and other information about Avalon. You can read Avalon’s SEC filings, including this proxy statement, over the Internet at the SEC’s website at http://www.sec.gov. The reports and other information filed by Avalon with the SEC are also available at Avalon’s website, which is http://www.avalon-globocare.com. Information on Avalon’s website is not part of this proxy statement.
If you would like additional copies of this proxy statement or if you have questions about the Acquisition or the proposals to be presented at the annual meeting, you should contact us by telephone or in writing:
Avalon GloboCare Corp.
4400 Route 9 South, Suite 3100
Freehold, New Jersey 07728
Telephone: (732) 780-4400
Attention: Secretary
128
F-1
LONLON BIOTECH LTD. AND SUBSIDIARIES
CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
F-2
LONLON BIOTECH LTD. AND SUBSIDIARIES
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020 and 2019
CONTENTS
F-3
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of Lonlon Biotech Ltd.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Lonlon Biotech Ltd. and its subsidiaries (collectively the Company) as of December 31, 2020 and 2019, and the related statements of operations and comprehensive loss, changes in shareholders’ equity, and cash flows for each of the years in the two-year period ended December 31, 2020, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020 and 2019, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Going Concern Matter
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, the Company has suffered significant losses from operations and had significant working capital deficit and accumulated deficit that raises substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Friedman LLP
We have served as the Company’s auditor since 2021.
New York, New York
June 24, 2021
F-4
LONLON BIOTECH LTD. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash | $ | 18,935 | $ | 164,994 | ||||
| Accounts receivable | 45,271 | 1,528 | ||||||
| Recoverable VAT | 335,150 | 320,247 | ||||||
| Inventory | 125,962 | 104,355 | ||||||
| Prepaid expenses and other current assets | 21,017 | 58,007 | ||||||
| Total Current Assets | 546,335 | 649,131 | ||||||
| NON-CURRENT ASSETS: | ||||||||
| Security deposit and other long-term assets | 50,012 | 75,843 | ||||||
| Operating lease right-of-use assets, net | 320,123 | 487,797 | ||||||
| Property and equipment, net | 2,567,522 | 3,110,094 | ||||||
| Total Non-current Assets | 2,937,657 | 3,673,734 | ||||||
| Total Assets | $ | 3,483,992 | $ | 4,322,865 | ||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Notes payable | $ | 918,752 | $ | - | ||||
| Notes payable - related party | 245,000 | - | ||||||
| Accounts payable | 310,330 | 79,439 | ||||||
| Salary payable | 105,810 | 107,014 | ||||||
| Accrued leasehold improvements liabilities | 315,583 | 1,428 | ||||||
| Accrued liabilities and other payables | 37,432 | 59,329 | ||||||
| Deferred revenue | 88,508 | 2,821 | ||||||
| Deferred grant income | 260,679 | 171,074 | ||||||
| Operating lease obligation | 155,470 | 133,133 | ||||||
| Total Current Liabilities | 2,437,564 | 554,238 | ||||||
| NON-CURRENT LIABILITIES: | ||||||||
| Deferred grant income - noncurrent portion | 351,677 | 495,651 | ||||||
| Operating lease obligation - noncurrent portion | 105,566 | 300,235 | ||||||
| Total Non-current Liabilities | 457,243 | 795,886 | ||||||
| Total Liabilities | 2,894,807 | 1,350,124 | ||||||
| Commitments and Contingencies | ||||||||
| SHAREHOLDERS’ EQUITY: | ||||||||
| Ordinary shares, $1.00 par value; 50,000 shares authorized; 10,001 shares issued and outstanding at December 2020 and 2019 * | 10,001 | 10,001 | ||||||
| Additional paid-in capital | 8,946,197 | 8,946,197 | ||||||
| Accumulated deficit | (8,380,014 | ) | (5,937,651 | ) | ||||
| Accumulated other comprehensive income (loss) | 13,001 | (45,806 | ) | |||||
| Total shareholders’ equity | 589,185 | 2,972,741 | ||||||
| Total Liabilities and Shareholders’ Equity | $ | 3,483,992 | $ | 4,322,865 | ||||
| * | The shares amounts are presented on a retroactive basis. |
The accompanying notes are an integral part of these consolidated financial statements.
F-5
LONLON BIOTECH LTD. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| REVENUES | ||||||||
| General laboratory testing | $ | 649,932 | $ | 77,594 | ||||
| Immunology and hematology testing | 440,183 | 15,736 | ||||||
| Total Revenues | 1,090,115 | 93,330 | ||||||
| COST OF REVENUES | ||||||||
| General laboratory testing | 343,794 | 35,972 | ||||||
| Immunology and hematology testing | 197,444 | 10,269 | ||||||
| Total Cost of Revenues | 541,238 | 46,241 | ||||||
| GROSS PROFIT | ||||||||
| General laboratory testing | 306,138 | 41,622 | ||||||
| Immunology and hematology testing | 242,739 | 5,467 | ||||||
| Total Gross Profit | 548,877 | 47,089 | ||||||
| OPERATING EXPENSES: | ||||||||
| Research and development expenses | 2,813,250 | 2,624,879 | ||||||
| Selling and marketing expenses | 163,145 | - | ||||||
| General and administrative expenses | 925,438 | 1,020,825 | ||||||
| Grant income | (929,505 | ) | (612,769 | ) | ||||
| Total Operating Expenses | 2,972,328 | 3,032,935 | ||||||
| LOSS FROM OPERATIONS | (2,423,451 | ) | (2,985,846 | ) | ||||
| OTHER (EXPENSE) INCOME | ||||||||
| Interest expense | (12,397 | ) | - | |||||
| Interest expense - related party | (11,169 | ) | - | |||||
| Other income | 4,654 | 6,942 | ||||||
| Total Other (Expense) Income, net | (18,912 | ) | 6,942 | |||||
| LOSS BEFORE INCOME TAXES | (2,442,363 | ) | (2,978,904 | ) | ||||
| INCOME TAXES | - | - | ||||||
| NET LOSS | $ | (2,442,363 | ) | $ | (2,978,904 | ) | ||
| COMPREHENSIVE LOSS: | ||||||||
| NET LOSS | $ | (2,442,363 | ) | $ | (2,978,904 | ) | ||
| OTHER COMPREHENSIVE INCOME (LOSS) | ||||||||
| Unrealized foreign currency translation gain (loss) | 58,807 | (50,204 | ) | |||||
| COMPREHENSIVE LOSS | $ | (2,383,556 | ) | $ | (3,029,108 | ) | ||
| NET LOSS PER ORDINARY SHARE: | ||||||||
| Basic and diluted * | $ | (244.21) | $ | (297.86) | ||||
| WEIGHTED AVERAGE ORDINARY SHARES OUTSTANDING: | ||||||||
| Basic and diluted * | 10,001 | 10,001 | ||||||
| * | The shares and per share amounts are presented on a retroactive basis. |
The accompanying notes are an integral part of these consolidated financial statements.
F-6
LONLON BIOTECH LTD. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CHANGES IN SHAEHOLDERS’ EQUITY
For the Years Ended December 31, 2020 and 2019
| Accumulated | ||||||||||||||||||||||||
| Ordinary Shares * | Additional | Other | Total | |||||||||||||||||||||
| Number of Shares | Amount | Paid-in Capital | Accumulated Deficit | Comprehensive Income (Loss) | Shareholders’ Equity | |||||||||||||||||||
| Balance as of December 31, 2018 | 10,001 | $ | 10,001 | $ | 8,946,197 | $ | (2,958,747 | ) | $ | 4,398 | $ | 6,001,849 | ||||||||||||
| Foreign currency translation adjustment | - | - | - | - | (50,204 | ) | (50,204 | ) | ||||||||||||||||
| Net loss for the year | - | - | - | (2,978,904 | ) | - | (2,978,904 | ) | ||||||||||||||||
| Balance as of December 31, 2019 | 10,001 | 10,001 | 8,946,197 | (5,937,651 | ) | (45,806 | ) | 2,972,741 | ||||||||||||||||
| Foreign currency translation adjustment | - | - | - | - | 58,807 | 58,807 | ||||||||||||||||||
| Net loss for the year | - | - | - | (2,442,363 | ) | - | (2,442,363 | ) | ||||||||||||||||
| Balance as of December 31, 2020 | 10,001 | $ | 10,001 | $ | 8,946,197 | $ | (8,380,014 | ) | $ | 13,001 | $ | 589,185 | ||||||||||||
| * | The shares amounts are presented on a retroactive basis. |
The accompanying notes are an integral part of these consolidated financial statements.
F-7
LONLON BIOTECH LTD. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | (2,442,363 | ) | $ | (2,978,904 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation | 1,147,115 | 889,410 | ||||||
| Amortization of right-of-use assets | 189,283 | 169,451 | ||||||
| Loss on disposal of property and equipment | 12,855 | 406 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivable | (41,306 | ) | (1,540 | ) | ||||
| Recoverable VAT | 5,973 | (93,654 | ) | |||||
| Inventory | (13,907 | ) | (92,778 | ) | ||||
| Prepaid expenses and other current assets | 38,647 | 124,134 | ||||||
| Security deposit and other long-term assets | 3,116 | - | ||||||
| Accounts payable | 213,553 | 34,799 | ||||||
| Salary payable | (7,849 | ) | 50,441 | |||||
| Accrued liabilities and other payables | (24,445 | ) | 34,546 | |||||
| Deferred revenue | 80,924 | 2,842 | ||||||
| Deferred grant income | (93,261 | ) | (157,859 | ) | ||||
| Operating lease obligation | (190,279 | ) | (173,647 | ) | ||||
| NET CASH USED IN OPERATING ACTIVITIES | (1,121,944 | ) | (2,192,353 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Purchase of property and equipment | (128,109 | ) | (870,110 | ) | ||||
| Prepayment for property and equipment | - | (11,578 | ) | |||||
| NET CASH USED IN INVESTING ACTIVITIES | (128,109 | ) | (881,688 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Proceeds from borrowings | 869,578 | - | ||||||
| Proceeds from related party’s borrowings | 608,704 | - | ||||||
| Repayments for related party’s borrowings | (376,817 | ) | - | |||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES | 1,101,465 | - | ||||||
| EFFECT OF EXCHANGE RATE ON CASH | 2,529 | (16,066 | ) | |||||
| NET DECREASE IN CASH | (146,059 | ) | (3,090,107 | ) | ||||
| CASH - beginning of year | 164,994 | 3,255,101 | ||||||
| CASH - end of year | $ | 18,935 | $ | 164,994 | ||||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | ||||||||
| Cash paid for: | ||||||||
| Interest | $ | 20,782 | $ | - | ||||
| NON-CASH INVESTING AND FINANCING ACTIVITIES: | ||||||||
| Property and equipment acquired on credit as payable | $ | 297,251 | $ | 1,439 | ||||
| Property and equipment acquired by decreasing prepayment for long-term assets | $ | 26,087 | $ | - | ||||
The accompanying notes are an integral part of these consolidated financial statements.
F-8
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – ORGANIZATION AND NATURE OF OPERATIONS
Lonlon Biotech Ltd. (“Senlang” or the “Company”) is a holding company incorporated in the British Virgin Islands (“BVI”) on October 15, 2020. The Company is mainly engaged in the business of research and development in relation to CAR-T cell therapy, immune cell therapy and related drug development in the People’s Republic of China (“PRC” or “China”), through a variable interest entity (“VIE” as defined in Note 4), Hebei Senlang Biotechnology Co., Ltd. (“SenlangBio”), which was established on January 29, 2016, and subsidiary of the VIE. SenlangBio also provides immunology and hematology testing services, mainly related to cell therapy, including tumor biomarkers and immunophenotyping. On February 9, 2018, SenlangBio formed a wholly owned subsidiary, Shijiazhuang Senlang Medical Laboratory Co., Ltd. (“SenlangBio Clinical Laboratory”), in China, which focuses on general laboratory testing for patients and other customers, including genomics, proteomics, routine blood/urine testing, COVID-19 PCR/antibody testing etc.
On November 2, 2020, Senlang established a wholly owned subsidiary in Hong Kong, Lonlon Biotech Investment Limited (“Senlang HK”), which is a holding company. On November 20, 2020, Senlang HK established a Wholly Foreign-Owned Enterprise in China, Beijing Langlang Runfeng Biotechnology Co., Ltd. (“Senlang BJ” or “WFOE”).
On April 26, 2021, Senlang BJ entered into a series of contractual arrangements, or VIE agreements with SenlangBio and 13 equity holders of SenlangBio, through which the Company obtained control and became the primary beneficiary of SenlangBio, hereinafter referred to as the Reorganization. As a result, SenlangBio became the Company’s VIE.
On April 26, 2021, the Company completed its reorganization of the entities under the common control of 13 majority shareholders through their 100% controlled entities incorporated in the British Virgin Islands, and indirectly owned a majority of the equity interests of the Company, its subsidiaries, its VIE and the VIE’s subsidiary prior to and after the Reorganization. The Company was established as a holding company of Senlang BJ. Senlang BJ is the primary beneficiary of SenlangBio, and all of these entities are under common control of the Company’s ultimate controlling shareholders before and after the Reorganization, which results in the consolidation of the Company and has been accounted for as a reorganization of entities under common control at carrying value and for accounting purpose, the reorganization was accounted for as a recapitalization. The consolidated financial statements are prepared on the basis as if the Reorganization became effective as of the beginning of the first period presented in the accompanying consolidated financial statements of the Company.
The following chart illustrates the Company’s corporate structure, including its subsidiaries, consolidated variable interest entity and VIE’s subsidiary as of the issuance date of this report:
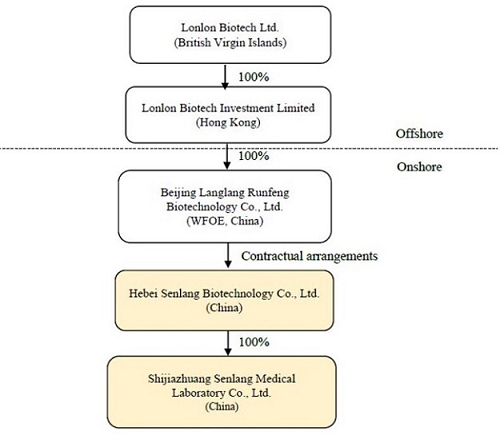
F-9
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – ORGANIZATION AND NATURE OF OPERATIONS (continued)
VIE Agreements with SenlangBio
Upon the completion of the reorganization, the Company, through the WFOE, entered into the following contractual arrangements with the VIE and the VIE’s 13 shareholders that enabled the Company to (1) have power to direct the activities that most significantly affects the economic performance of the VIE, and (2) receive the economic benefits of the VIE that could be significant to the VIE. Accordingly, the WFOE was considered the primary beneficiary of the VIE and had consolidated the VIE and the VIE’s subsidiary’ financial results of operations, assets and liabilities in the Company’s consolidated financial statements.
Contracts that give the Company effective control of the VIE
Equity Pledge Agreement
Under the Equity Pledge Agreement between Senlang BJ, SenlangBio and SenlangBio’s shareholders, SenlangBio’s shareholders agree to pledge all of their equity interests in SenlangBio to Senlang BJ to guarantee the performance of SenlangBio and SenlangBio’s shareholders’ obligations under the Exclusive Technical Consultation and Service Agreement, the Exclusive Purchase Option Agreement, the Shareholder’s Rights Proxy Agreement, and the Spousal Consent (“Transaction Agreements”). Under the terms of the Equity Pledge Agreement, in the event that SenlangBio or SenlangBio’s shareholders breach their respective contractual obligations under the Transaction Agreements or the Equity Pledge Agreement, Senlang BJ, as pledgee, is entitled to directly exercise the pledge right and to notify SenlangBio’s shareholders to immediately repay or pay the loans or other payables under the Transaction Agreements. SenlangBio’s shareholders further agreed not to dispose of the pledged equity interests without prior written consent from Senlang BJ.
The pledge is effective on the date when the registration of the equity pledge is completed with the competent administration for industry and commerce, and the term of validity of the pledge is the same as the longest term of validity in the Transaction Agreements.
The purposes of the Equity Pledge Agreement are to (1) guarantee the performance of SenlangBio and SenlangBio’s shareholders’ obligations under the Transaction Agreements, (2) make sure SenlangBio’s shareholders do not transfer or assign the pledged equity interests, or create or allow any encumbrance that would prejudice interests of Senlang BJ without prior written consent from Senlang BJ, and (3) provide Senlang BJ control over SenlangBio.
In the event SenlangBio or SenlangBio’s shareholders breaches their contractual obligations under the Transaction Agreements or Equity Pledge Agreement, Senlang BJ will be entitled to (1) be compensated on a preferential basis with the proceeds from the conversion, auction or sale of the pledged equity, and (2) notify SenlangBio’s shareholders to immediately repay the loans or other payables under the Transaction Agreements.
Exclusive Purchase Option Agreement
Under the Exclusive Purchase Option Agreement, SenlangBio’s shareholders irrevocably grants Senlang BJ (or its designee) an exclusive right to purchase the equity held by SenlangBio’s shareholders in the SenlangBio in whole or in part at any time during the term of the Agreement; SenlangBio also irrevocably grants Senlang BJ (or its designee) an exclusive right to purchase the assets owned by SenlangBio in whole or in part at any time during the term of the Agreement.
With respect to consideration for equity purchase, Senlang BJ has the right to purchase all or part of the equity held by SenlangBio’s shareholders in SenlangBio at the lowest price permitted by the PRC laws. With respect to the price for asset purchase, Senlang BJ has the right to purchase SenlangBio’s assets at a price equivalent of the net book value of the purchased assets; provided that if the minimum price permitted by the PRC law is higher than the net book value, the minimum price permitted by the PRC laws will prevail.
Under the Exclusive Purchase Option Agreement, Senlang BJ may purchase all or part of the equity held by SenlangBio’s shareholders in SenlangBio and all or part of the assets owned by SenlangBio at any time to the extent permitted by PRC laws. The Exclusive Purchase Option Agreement, together with the Equity Pledge Agreement, Exclusive Technical Consultation and Service Agreement, and the Proxy Agreement, enable Senlang BJ to exercise effective control over SenlangBio. The Exclusive Purchase Option Agreement will become effective upon the affixation of signatures or corporate seals by the Senlang BJ, SenlangBio and SenlangBio’s shareholders, and will remain in effect, unless terminated by Senlang BJ by giving a thirty (30) day advance written notice.
F-10
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – ORGANIZATION AND NATURE OF OPERATIONS (continued)
Contracts that give the Company effective control of the VIE
Shareholder’s Rights Proxy Agreement
Under the Shareholder’s Rights Proxy Agreement, SenlangBio’s shareholders authorize any entity or individual designated by Senlang BJ to act on their behalf as their exclusive agent to exercise all shareholder’s rights under the PRC laws and the Articles of Association, including but not limited to: (a) to convene, attend and vote on all the matters during shareholders’ meetings; (b) to transfer, pledge or dispose of, or create encumbrance on the equity; (c) to receive dividends; (d) to participate in judicial proceedings or execute legal documents in relation to shareholders’ rights; (e) to appoint legal representatives, directors and officers of SenlangBio; and (f) to enter into contracts and exercise the Exclusive Purchase Option Agreement.
The Shareholder’s Rights Proxy Agreement shall remain effective until the earlier of: (a) the date on which SenlangBio’s shareholders are no longer the nominee or actual shareholders of Senlang BJ; (b) the date on which Senlang BJ requests the proxy to be terminated in writing; or (c) the date on which the assets and licenses of SenlangBio have been fully transferred to Senlang BJ.
Contracts that enable the Company to receive substantially all of the economic benefits from the VIE
Exclusive Technical Consultation and Service Agreement
Pursuant to the Exclusive Technical Consultation and Service Agreement between Senlang BJ and SenlangBio, Senlang BJ provides SenlangBio with technical consultation and services, including conducting market research, assisting with developing management and sales plans, implementing relevant technology application, and providing other consultation services for computer network, finance, business, legal affairs, operation, human resources, and other aspects of SenlangBio. Additionally, Senlang BJ agrees to grant SenlangBio its trademarks, software copyrights, management systems, management methods and other intellectual property in relation to its services on a chargeable and revocable basis, but such grant shall not result in the transfer of any intellectual property or create any restriction on Senlang BJ’s full ownership.
For services rendered to SenlangBio under this agreement, Senlang BJ is entitled to collect a service fee calculated based on the complexity of services rendered, time required by Senlang BJ, and the exact contents and commercial value of services rendered. During the term of this agreement, Senlang BJ shall enjoy all the economic benefits derived from SenlangBio’s operation, and in the event of serious difficulties in SenlangBio’s operations, Senlang BJ may provide SenlangBio with financial support, and Senlang BJ has the right to request SenlangBio to cease operation. The Exclusive Technical Consultation and Service Agreement shall remain in effect for ten (10) years and shall be automatically renewed unless it is terminated earlier by Senlang BJ.
Based on the foregoing VIE Agreements, Senlang BJ has effective control of SenlangBio which enables Senlang BJ to receive all of the expected residual returns and absorb the expected losses of the VIE and its subsidiary. Management therefore concludes that the Company, through the above contractual arrangements, has the power to direct the activities that most significantly impact the VIE’s economic performance, bears the risks of and enjoys the rewards normally associated with ownership of the VIE, and therefore the Company is the ultimate primary beneficiary of the VIE. Consequently, the Company consolidates the accounts of SenlangBio and its subsidiary for the periods presented herein, in accordance with Accounting Standards Codification (“ASC”) 810-10, Consolidation.
The accompanying consolidated financial statements reflect the activities of Senlang and each of the following entities:
| Name | Background | Ownership | ||
| Subsidiaries: | ||||
| Senlang HK | A Hong Kong company | 100% owned by Senlang | ||
| Incorporated on November 2, 2020 | ||||
| Senlang BJ | A PRC limited liability company and a wholly foreign owned enterprise | 100% owned by Senlang HK | ||
| Incorporated on November 20, 2020 | ||||
| VIE: | ||||
| SenlangBio | A PRC limited liability company | VIE | ||
| Incorporated on January 29, 2016 Immunology and hematology testing service provider | ||||
| VIE’s subsidiary: | ||||
| SenlangBio | A PRC limited liability company | 100% owned by SenlangBio | ||
| Clinical Laboratory | Incorporated on February 9, 2018 | |||
| General laboratory testing service provider |
F-11
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2 – BASIS OF PRESENTATION AND GOING CONCERN AND LIQUIDITY
Basis of Presentation
The accompanying consolidated financial statements and related notes have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and with the rules and regulations of the U.S. Securities and Exchange Commission for financial information.
The Company’s consolidated financial statements include the accounts of the Company and its subsidiaries, VIE and subsidiary of VIE over which the Company exercises control and, when applicable, entity for which the Company has a controlling financial interest or is the primary beneficiary. All significant intercompany accounts and transactions have been eliminated in consolidation.
Going Concern and Liquidity
The Company currently has limited operations. Currently, the Company’s operations are focused on utilizing cell and gene engineering technologies to generate innovative and transformative cellular immunotherapies for solid and hematologic cancers. The Company provides general laboratory testing and immunology and hematology testing services for patients and other customers in China. These consolidated financial statements have been prepared assuming that the Company will continue as a going concern, which contemplates, among other things, the realization of assets and the satisfaction of liabilities in the normal course of business.
As reflected in the accompanying consolidated financial statements, the Company had a working capital deficit and accumulated deficit of $1,891,229 and $8,380,014, respectively, at December 31, 2020, has incurred net loss of $2,442,363 and $2,978,904, respectively, for the years ended December 31, 2020 and 2019, and generated negative cash flow from operating activities of $1,121,944 and $2,192,353, respectively, for the year ended December 31, 2020 and 2019. The Company has a limited operating history and its continued growth is dependent upon the continuation of providing general laboratory testing and immunology and hematology testing services, and obtaining additional financing to fund future obligations and pay liabilities arising from normal business operations. In addition, the current cash balance cannot be projected to cover the operating expenses for the next twelve months from the release date of this report. These matters raise substantial doubt about the Company’s ability to continue as a going concern. The ability of the Company to continue as a going concern is dependent on the Company’s ability to raise additional capital, implement its business plan, and generate significant revenues. There are no assurances that the Company will be successful in its efforts to generate significant revenues, maintain sufficient cash balance or report profitable operations or to continue as a going concern. The Company plans on raising capital through bank and other borrowings to implement its business plan. However, there is no assurance these plans will be realized and that any additional financings will be available to the Company on satisfactory terms and conditions, if any.
The occurrence of an uncontrollable event such as the COVID-19 pandemic could negatively impact the Company’s operations even though the pandemic did not significantly impact the Company’s operation in 2020. However, given the dynamic nature of these circumstances, the uncertainty around the potential resurgence of the COVID-19 cases in China, and the instability of local policies and restrictions, the COVID-19 impact over the Company’s business in the year of 2021 cannot be reasonably estimated at this time. If COVID-19 cases resurged in the area the Company conducted its business and local government implemented new restrictions in the effort to contain the spread, it is expected the Company’s business will be negatively impacted.
The accompanying consolidated financial statements do not include any adjustments related to the recoverability or classification of asset-carrying amounts or the amounts and classification of liabilities that may result should the Company be unable to continue as a going concern.
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Use of Estimates and Assumptions
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from these estimates. Significant estimates during the years ended December 31, 2020 and 2019 include the useful lives of property and equipment, assumptions used in assessing impairment of long-term assets, and valuation of deferred tax assets and the associated valuation allowances.
F-12
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Fair Value of Financial Instruments and Fair Value Measurements
The Company adopted the guidance of Accounting Standards Codification (“ASC”) 820 for fair value measurements which clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
| ● | Level 1-Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date. |
| ● | Level 2-Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other than quoted prices that are observable, and inputs derived from or corroborated by observable market data. |
| ● | Level 3-Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information. |
The carrying values of current assets and current liabilities in the Company’s consolidated balance sheets approximated their fair values as of December 31, 2020 and 2019 due to their short-term nature.
ASC 825-10 “Financial Instruments”, allows entities to voluntarily choose to measure certain financial assets and liabilities at fair value (fair value option). The fair value option may be elected on an instrument-by-instrument basis and is irrevocable, unless a new election date occurs. If the fair value option is elected for an instrument, unrealized gains and losses for that instrument should be reported in earnings at each subsequent reporting date. The Company did not elect to apply the fair value option to any outstanding instruments.
Cash
Cash consists of cash on hand and cash in the bank. The Company maintains cash with financial institutions in the PRC.
Credit Risk and Uncertainties
The Company’s cash is maintained with state-owned banks within the PRC. Balances at state-owned banks within the PRC are covered by insurance up to RMB 500,000 (approximately $77,000) per bank. Any balance over RMB 500,000 per bank in PRC will not be covered. At December 31, 2020, cash balances held in the PRC are RMB 123,659 (approximately $19,000). The Company has not experienced any losses in such accounts and believes it is not exposed to any risks on its cash in bank accounts.
The Company’s operations are carried out in PRC. Accordingly, the Company’s business, financial condition and results of operations may be influenced by the political, economic and legal environment in the PRC, and by the general state of the PRC’s economy. The Company’s operations in PRC are subject to specific considerations and significant risks not typically associated with companies in North America. The Company’s results may be adversely affected by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
Financial instruments which potentially subject the Company to concentrations of credit risk consist principally of trade accounts receivable. A portion of the Company’s sales are credit sales which is to the customer whose ability to pay is dependent upon the industry economics prevailing in Hebei province, China; however, concentrations of credit risk with respect to trade accounts receivable is limited due to short term payment terms. The Company also performs ongoing credit evaluations of its customers to help further reduce credit risk.
F-13
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable are presented net of an allowance for doubtful accounts. The Company maintains allowances for doubtful accounts for estimated losses. The Company reviews the accounts receivable on a periodic basis and makes general and specific allowances when there is doubt as to the collectability of individual balances. In evaluating the collectability of individual receivable balances, the Company considers many factors, including the age of the balance, a customer’s payment history, its current credit-worthiness and current economic trends. Accounts are written off after exhaustive efforts at collection.
Management believes that the accounts receivable are fully collectable. Therefore, no allowance for doubtful accounts is deemed to be required on its accounts receivable at December 31, 2020 and 2019. The Company historically has not experienced significant uncollectible accounts receivable.
Inventory
Inventory consists of raw materials. Inventory is stated at the lower of cost or net realizable value. Cost is determined using the first-in, first-out (FIFO) method. A reserve is established when management determines that certain inventory may not be saleable. If inventory costs exceed expected net realizable value due to obsolescence or quantities in excess of expected demand, the Company will record reserves for the difference between the cost and the estimated net realizable value. The Company did not record any inventory reserve at December 31, 2020 and 2019.
Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets primarily consist of prepaid utilities and prepaid internet service fees, which are recognized as expenses over the related service periods. As of December 31, 2020 and 2019, prepaid expenses and other current assets amounted to $21,017 and $58,007, respectively.
Property and Equipment
Property and equipment are carried at cost and are depreciated on a straight-line basis over the estimated useful lives of the assets. The estimated useful lives are as follows:
| Useful Life | Estimated Residual Value Rate | |||
| Laboratory equipment | 3 - 5 Years | 5% | ||
| Office equipment and furniture | 3 - 5 Years | 5% | ||
| Vehicles | 4 - 5 Years | 5% | ||
| Leasehold improvements | Shorter of the remaining lease terms or estimated useful life | 0% | ||
| Software | 1 - 2 Years | 0% |
The cost of repairs and maintenance is expensed as incurred; major replacements and improvements are capitalized. When assets are retired or disposed of, the cost and accumulated depreciation are removed from the accounts, and any resulting gains or losses are included in income or loss in the period of disposition. The Company examines impairment of fixed assets when events or changes in circumstances reflect the fact that their recorded value may not be recoverable.
Impairment of Long-lived Assets
In accordance with ASC Topic 360, the Company reviews long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable, or at least annually. The Company recognizes an impairment loss when the sum of expected undiscounted future cash flows is less than the carrying amount of the asset. The amount of impairment is measured as the difference between the asset’s estimated fair value and its book value. There were no triggering events requiring assessment of impairment as of December 31, 2020 and 2019. For the years ended December 31, 2020 and 2019, no impairment of long-lived assets was recognized.
F-14
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Government Grants and Deferred Grant Income
Government grants related to purchased long-live assets, most of which are laboratory equipment for research and development, are recorded as deferred grant income initially and recognized as grant income on a systematic basis over the useful lives of the assets. Government grants that are to compensate incurred costs, expenses or losses are recognized in the current period. The Company applies the presentation method consistently to the similar government grants in the consolidated financial statements. Government grants that are related to operating activities are included in operating income (loss), otherwise, they are recorded in other income (expense).
Grants and subsidies received from Chinese government are recognized when the proceeds are received or collectible and related milestones have been reached and all contingencies have been resolved. For the years ended December 31, 2020 and 2019, grant income amounted to $929,505 and $612,769, respectively.
Deferred grant income represents grants collected but not earned as of each of the balance sheet date. This is primarily composed of receipts of the government subsidies for acquisition of lab equipment and reimbursement on office rent and research and development expense. Deferred grant income was recognized as grant income in accordance with aforementioned methodology. As of December 31, 2020 and 2019, deferred grant income amounted to $612,356 and $666,725, respectively.
Value Added Tax
The Company is subject to value added tax (“VAT”) on revenues earned for services provided in the PRC. The amount of VAT liability is determined by applying the applicable tax rates to the invoiced amount of services provided (output VAT) less VAT paid on purchases made with the relevant supporting invoices (input VAT). The Company reports revenue net of PRC’s value added tax for all the periods presented in the consolidated statements of operations and comprehensive loss.
Accrued Liabilities and Other Payables
Accrued liabilities and other payables primarily consist of accrued and unpaid interest related to notes payable, taxes payable, and accrued liabilities for other miscellaneous items. As of December 31, 2020 and 2019, accrued liabilities and other payables amounted to $37,432 and $59,329, respectively.
Deferred Revenue
Payments received prior to services being performed are recorded as deferred revenue until such time as the services are performed. As of December 31, 2020 and 2019, deferred revenue amounted to $88,508 and $2,821, respectively.
Revenue Recognition
Effective January 1, 2018, the Company began recognizing revenue under Accounting Standards Codification (“ASC”) Topic 606, Revenue from Contracts with Customers (“ASC 606”), using the modified retrospective transition method. The impact of adopting the new revenue standard was not material to the Company’s consolidated financial statements and there was no adjustment to beginning accumulated deficit on January 1, 2018. The core principle of the revenue standard is that a company should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. The following five steps are applied to achieve that core principle:
| ● | Step 1: Identify the contract with the customer |
| ● | Step 2: Identify the performance obligations in the contract |
| ● | Step 3: Determine the transaction price |
| ● | Step 4: Allocate the transaction price to the performance obligations in the contract |
| ● | Step 5: Recognize revenue when the company satisfies a performance obligation |
F-15
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Revenue Recognition (continued)
In order to identify the performance obligations in a contract with a customer, a company must assess the promised goods or services in the contract and identify each promised goods or service that is distinct. A performance obligation meets ASC 606’s definition of a “distinct” goods or service (or bundle of goods or services) if both of the following criteria are met:
| ● | The customer can benefit from the goods or service either on its own or together with other resources that are readily available to the customer (i.e., the goods or service is capable of being distinct). |
| ● | The entity’s promise to transfer the goods or service to the customer is separately identifiable from other promises in the contract (i.e., the promise to transfer the goods or service is distinct within the context of the contract). |
If a goods or service is not distinct, the goods or service is combined with other promised goods or services until a bundle of goods or services is identified that is distinct.
The transaction price is the amount of consideration to which an entity expects to be entitled in exchange for transferring promised goods or services to a customer, excluding amounts collected on behalf of third parties (for example, some sales taxes). The consideration promised in a contract with a customer may include fixed amounts, variable amounts, or both. Variable consideration is included in the transaction price only to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved.
The transaction price is allocated to each performance obligation on a relative standalone selling price basis. The transaction price allocated to each performance obligation is recognized when that performance obligation is satisfied, at a point in time or over time as appropriate.
The Company’s revenues are derived from providing general laboratory testing services and immunology and hematology testing services for patients and other customers. Revenues related to its service offerings are recognized at a point in time when service is rendered. Any payments received in advance of the performance of services are recorded as deferred revenue until such time as the services are performed. Except for deferred revenue, the Company did not have any other contract liability nor contract asset as of December 31, 2020 and 2019.
The Company does not offer promotional payments, customer coupons, rebates or other cash redemption offers to its customers.
The Company has concluded that its government grants are not within the scope of ASC Topic 606 as they do not meet the definition of a contract with a customer. The Company has concluded that the grants meet the definition of a contribution and are non-reciprocal transactions, and has also concluded that Subtopic 958-605, Not-for-Profit-Entities-Revenue Recognition does not apply, as it is a business entity and the grants are with governmental agencies.
In the absence of applicable guidance under US GAAP, effective January 1, 2018, the Company developed a policy for the recognition of grant revenue when the related costs are incurred or the related long-live assets are purchased, and the right to payment is realized.
The Company believes this policy is consistent with the overarching premise in ASC Topic 606, to ensure that revenue recognition reflects the transfer of promised goods or services to customers in an amount that reflects the consideration that it expects to be entitled to in exchange for those goods or services, even though there is no exchange as defined in ASC Topic 606.
The Company believes the recognition of revenue as costs are incurred or long-live assets are purchased, and amounts become realizable is analogous to the concept of transfer of control of a service over time under ASC Topic 606.
F-16
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Disaggregation of Revenues
In the following table, revenues are disaggregated by segment for the years ended December 31, 2020 and 2019:
| Revenue Stream | 2020 | 2019 | Revenue Stream Detail | |||||||
| General laboratory testing | $ | 649,932 | $ | 77,594 | Providing general laboratory testing services, including genomics, proteomics, routine blood/urine testing, COVID-19 PCR/antibody testing etc., to patients and other customers. | |||||
| Immunology and hematology testing | 440,183 | 15,736 | Providing immunology and hematology testing services, mainly related to cell therapy, including tumor biomarkers and immunophenotyping, to customers. | |||||||
| Total revenue | $ | 1,090,115 | $ | 93,330 | ||||||
Operating Lease
In February 2016, the Financial Accounting Standards Board (the “FASB”) issued Accounting Standards Update (“ASU”) 2016-02, Leases (Topic 842). Lessees are required to recognize a right-of-use asset and a lease liability for virtually all of their leases (other than leases that meet the definition of a short-term lease). The liability is equal to the present value of lease payments. The asset is based on the liability, subject to certain adjustments, such as for initial direct costs. For income statement purposes, a dual model was retained, requiring leases to be classified as either operating or finance leases. Operating leases result in straight-line expense (similar to operating leases under the prior accounting standard) while finance leases result in a front-loaded expense pattern (similar to capital leases under the prior accounting standard). Lessor accounting is similar to the prior model but updated to align with certain changes to the lessee model (e.g., certain definitions, such as initial direct costs, have been updated) and the new revenue standard, ASU 2014-9.
The Company adopted this new accounting standard on January 1, 2018 on a modified retrospective basis and applied the new standard to leases through a cumulative-effect adjustment to beginning accumulated deficit. As a result, comparative financial information has not been restated and continues to be reported under the accounting standards in effect for those periods. The Company elected to adopt both the transition relief provided in ASU 2018-11 and the package of practical expedients which allowed it, among other things, to retain historical lease classifications and accounting for any leases that existed prior to adoption of the standard. Additionally, the Company elected the practical expedients allowing it not to separate lease and non-lease components and not record leases with an initial term of twelve months or less (“short-term leases”) on the balance sheets across all existing asset classes. The new standard had a material impact on the consolidated balance sheets but did not materially impact the Company’s consolidated operating results and had no impact on the Company’s beginning accumulated deficit and cash flows. The following is a discussion of the Company’s lease policy under the new lease accounting standard:
The Company determines if an arrangement contains a lease at the inception of a contract. Right-of-use assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising from the lease. Right-of- use assets and lease liabilities are recognized at the commencement date based on the present value of the remaining future minimum lease payments. As the interest rate implicit in the Company’s leases is not readily determinable, the Company utilizes its borrowing rates set by the Central Bank of the People’s Republic of China, determined by class of underlying asset, to discount the lease payments.
The Company leases premises for offices under non-cancellable operating leases. Operating lease payments are expensed over the term of lease. The Company leases do not include options to extend nor any restrictions or covenants. The Company has historically been able to renew its office leases. Under the terms of the lease agreements, the Company has no legal or contractual asset retirement obligations at the end of the lease.
Impact of New Lease Standard on Balance Sheet Line Items
As a result of applying the new lease standard using a modified retrospective method, the Company recognized operating lease right-of-use assets of $799,983, current portion of operating lease liabilities of $150,985, and long-term operating lease liabilities of $648,998 as of January 1, 2018. There is no impact on equity as of January 1, 2018, the date of adoption. See Note 13.
F-17
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
General Laboratory Testing Services Costs
Costs of general laboratory testing services include the cost of inventory, labor and related benefits, depreciation, and other overhead costs.
Immunology and Hematology Testing Services Costs
Costs of immunology and hematology testing services include the cost of inventory, labor and related benefits, depreciation, and other overhead costs.
Employee Benefits
The full-time employees of the Company are entitled to staff welfare benefits including medical care, housing fund, pension benefits, unemployment insurance and other welfare, which are government mandated defined contribution plans. The Company is required to accrue for these benefits based on certain percentages of the employees’ respective salaries, subject to certain ceilings, in accordance with the relevant PRC regulations, and make cash contributions to the state-sponsored plans out of the amounts accrued. Total expenses for the plans were $135,231 and $221,232 for the years ended December 31, 2020 and 2019, respectively, which were charged to the same accounts as the related salary costs in the same period as the related salary costs incurred.
Research and Development
Expenditures for research and product development costs are expensed as incurred. The Company incurred research and development expense of $2,813,250 and $2,624,879 in the years ended December 31, 2020 and 2019, respectively.
Income Taxes
The Company accounts for income taxes using the asset/liability method prescribed by ASC 740, “Income Taxes.” Under this method, deferred tax assets and liabilities are determined based on the difference between the financial reporting and tax bases of assets and liabilities using enacted tax rates that will be in effect in the period in which the differences are expected to reverse. The Company records a valuation allowance to offset deferred tax assets if, based on the weight of available evidence, it is more-likely-than-not that some portion, or all, of the deferred tax assets will not be realized. The effect on deferred taxes of a change in tax rates is recognized as income or loss in the period that includes the enactment date.
The Company follows the accounting guidance for uncertainty in income taxes using the provisions of ASC 740 “Income Taxes”. Using that guidance, tax positions initially need to be recognized in the financial statements when it is more likely than not the position will be sustained upon examination by the tax authorities. As of December 31, 2020 and 2019, the Company had no significant uncertain tax positions that qualify for either recognition or disclosure in the financial statements. Tax year that remains subject to examination is the years ended December 31, 2016 through December 31, 2020. The Company recognizes interest and penalties related to significant uncertain income tax positions in income tax expense. However, no such interest and penalties were recorded as of December 31, 2020 and 2019.
Loss per share
The Company computes loss per share in accordance with ASC 260, “Earnings per Share” (“ASC 260”). ASC 260 requires companies with complex capital structures to present basic and diluted earnings per share (“EPS”). Basic EPS is measured as net income divided by the weighted average common shares outstanding for the period. Diluted EPS presents the dilutive effect on a per share basis of potential common shares (e.g. convertible securities, options and warrants) as if they had been converted at the beginning of the periods presented, or issuance date, if later. Potential common shares that have an anti-dilutive effect (i.e. those that increase income per share or decrease loss per share) are excluded from the calculation of diluted EPS. As of December 31, 2020 and 2019, there were no dilutive shares.
Foreign Currency Translation
The reporting currency of the Company is U.S. Dollars. The functional currency of the parent company, Senlang, and Senlang HK, is the U.S. dollar and the functional currency of Senlang BJ, SenlangBio, and SenlangBio Clinical Laboratory is the Chinese Renminbi (“RMB”).
Monetary assets and liabilities denominated in currencies other than the reporting currency are translated into the reporting currency at the rates of exchange prevailing at the balance sheet date. Revenue and expenses are translated using average rates during each reporting period, and shareholders’ equity is translated at historical exchange rates. Cash flows are also translated at average translation rates for the periods, therefore, amounts reported on the statement of cash flows will not necessarily agree with changes in the corresponding balances on the consolidated balance sheet. Translation adjustments resulting from the process of translating the local currency financial statements into U.S. dollars are included in determining comprehensive income/loss. Transactions denominated in foreign currencies are translated into the functional currency at the exchange rates prevailing on the transaction dates. Assets and liabilities denominated in foreign currencies are translated into the functional currency at the exchange rates prevailing at the balance sheet date with any transaction gains and losses that arise from exchange rate fluctuations on transactions denominated in a currency other than the functional currency are included in the results of operations as incurred.
F-18
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Foreign Currency Translation (continued)
All of the Company’s revenue transactions are transacted in the functional currency of the Company. The Company does not enter into any material transaction in foreign currencies. Transaction gains or losses have not had, and are not expected to have, a material effect on the results of operations of the Company.
Asset and liability accounts at December 31, 2020 and 2019 were translated at 6.5306 RMB and 6.9632 RMB to $1.00, respectively, which were the exchange rates on the balance sheet dates. Equity accounts were stated at their historical rates. The average translation rates applied to the statements of operations for the years ended December 31, 2020 and 2019 were 6.8999 RMB and 6.9099 RMB to $1.00, respectively. Cash flows from the Company’s operations are calculated based upon the local currencies using the average translation rate.
Comprehensive Loss
Comprehensive loss is comprised of net loss and all changes to the statements of equity, except those due to investments by shareholders, changes in paid-in capital and distributions to shareholders. For the Company, comprehensive loss for the years ended December 31, 2020 and 2019 consisted of net loss and unrealized gain/loss from foreign currency translation adjustment.
Commitment and Contingencies
In the normal course of business, the Company is subject to contingencies, such as legal proceedings and claims arising out of its business, that cover a wide range of matters. Liabilities for such contingencies are recorded when it is probable that a liability has been incurred and the amount of the assessment can be reasonably estimated.
Segment Reporting
The Company uses “the management approach” in determining reportable operating segments. The management approach considers the internal organization and reporting used by the Company’s chief operating decision maker for making operating decisions and assessing performance as the source for determining the Company’s reportable segments. The Company’s chief operating decision maker is its Chief Executive Officer (“CEO”), who reviews operating results to make decisions about allocating resources and assessing performance for the entire Company. The Company has determined that it has two reportable business segments: general laboratory testing segment, and immunology and hematology testing segment. These reportable segments offer different types of services and products, have different types of revenue, and are managed separately as each requires different operating strategies and management expertise.
Related Parties
Parties are considered to be related to the Company if the parties, directly or indirectly, through one or more intermediaries, control, are controlled by, or are under common control with the Company. Related parties also include principal owners of the Company, its management, members of the immediate families of principal owners of the Company and its management and other parties with which the Company may deal with if one party controls or can significantly influence the management or operating policies of the other to an extent that one of the transacting parties might be prevented from fully pursuing its own separate interests. The Company discloses all significant related party transactions.
Recent Accounting Standards
In August 2018, the FASB issued ASU No. 2018-13, Fair Value Measurement (Topic 820): Disclosure Framework-Changes to the Disclosure Requirements for Fair Value Measurement. The objective of ASU 2018-13 is to improve the effectiveness of disclosures in the notes to the financial statements by removing, modifying, and adding certain fair value disclosure requirements to facilitate clear communication of the information required by generally accepted accounting principles. The amendments are effective for all entities for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2019 with early adoption permitted upon issuance of this ASU. The adoption of ASU 2018 – 13 did not have a material impact on the Company’s consolidated financial statements.
F-19
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Recent Accounting Standards (continued)
In June 2016, the FASB issued ASU 2016-13, Financial Instruments - Credit Losses (“Topic 326”). The ASU introduces a new accounting model, the Current Expected Credit Losses model (“CECL”), which requires earlier recognition of credit losses and additional disclosures related to credit risk. The CECL model utilizes a lifetime expected credit loss measurement objective for the recognition of credit losses at the time the financial asset is originated or acquired. ASU 2016-13 is effective for annual period beginning after December 15, 2022, including interim reporting periods within those annual reporting periods. The Company expects that the adoption will not have a material impact on the Company’s consolidated financial statements.
In December 2019, the FASB issued ASU 2019-12, Simplifying the Accounting for Income Taxes, as part of its Simplification Initiative to reduce the cost and complexity in accounting for income taxes. This standard removes certain exceptions related to the approach for intra period tax allocation, the methodology for calculating income taxes in an interim period and the recognition of deferred tax liabilities for outside basis differences. It also amends other aspects of the guidance to help simplify and promote consistent application of GAAP. The guidance is effective for interim and annual periods beginning after December 15, 2020, with early adoption permitted. The Company is evaluating the effects that the adoption of this guidance will have its consolidated financial statements.
Other accounting standards that have been issued or proposed by FASB that do not require adoption until a future date are not expected to have a material impact on the consolidated financial statements upon adoption. The Company does not discuss recent pronouncements that are not anticipated to have an impact on or are unrelated to its consolidated financial condition, results of operations, cash flows or disclosures.
NOTE 4 – VARIABLE INTEREST ENTITY AND OTHER CONSOLIDATION MATTERS
On April 26, 2021, Senlang BJ entered into VIE Agreements with SenlangBio and 13 shareholders of SenlangBio. The key terms of these VIE Agreements are summarized in “NOTE 1 – ORGANIZATION AND NATURE OF OPERATIONS” above. As a result of the VIE Agreements, the Company classifies SenlangBio as a VIE.
A VIE is an entity that has either a total equity investment that is insufficient to permit the entity to finance its activities without additional subordinated financial support, or whose equity investors lack the characteristics of a controlling financial interest, such as through voting rights, right to receive the expected residual returns of the entity or obligation to absorb the expected losses of the entity. The variable interest holder, if any, that has a controlling financial interest in a VIE is deemed to be the primary beneficiary and must consolidate the VIE. Senlang BJ is deemed to have a controlling financial interest and be the primary beneficiary of SenlangBio, because it has both of the following characteristics:
| 1. | Power to direct activities of a VIE that most significantly impact the entity’s economic performance, and |
| 2. | Obligation to absorb losses of the entity that could potentially be significant to the VIE or right to receive benefits from the entity that could potentially be significant to the VIE. |
Pursuant to the VIE Agreements, SenlangBio pays service fees equal to all of its net income to Senlang BJ. At the same time, Senlang BJ is entitled to receive all of SenlangBio’s expected residual returns. The VIE Agreements are designed so that SenlangBio operates for the benefit of the Company. Accordingly, the accounts of SenlangBio are consolidated in the accompanying financial statements pursuant to ASC 810-10, Consolidation. In addition, its financial positions and results of operations are included in the Company’s consolidated financial statements.
In addition, as all of these VIE agreements are governed by PRC law and provide for the resolution of disputes through arbitration in the PRC, they would be interpreted in accordance with PRC law and any disputes would be resolved in accordance with PRC legal procedures. The legal environment in the PRC is not as developed as in other jurisdictions, such as the United States. As a result, uncertainties in the PRC legal system could further limit the Company’s ability to enforce these VIE agreements. Furthermore, these contracts may not be enforceable in China if PRC government authorities or courts take a view that such contracts contravene PRC laws and regulations or are otherwise not enforceable for public policy reasons. In the event the Company is unable to enforce these VIE agreements, it may not be able to exert effective control over SenlangBio and its ability to conduct its business may be materially and adversely affected.
F-20
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 4 – VARIABLE INTEREST ENTITY AND OTHER CONSOLIDATION MATTERS (continued)
All of the Company’s main current operations are conducted through SenlangBio and subsidiary of SenlangBio. Current regulations in China permit SenlangBio to pay dividends to the Company only out of its accumulated distributable profits, if any, determined in accordance with its article of association and PRC accounting standards and regulations. The ability of SenlangBio to make dividends and other payments to the Company may be restricted by factors including changes in applicable foreign exchange and other laws and regulations.
The following consolidated financial information of the VIE and VIE’s subsidiary as a whole as of December 31, 2020 and 2019 and for the years ended December 31, 2020 and 2019 was included in the accompanying consolidated financial statements of the Company. Transactions between the VIE and VIE’s subsidiary are eliminated in the financial information presented below:
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| Cash | $ | 18,935 | $ | 164,994 | ||||
| Accounts receivable | 45,271 | 1,528 | ||||||
| Recoverable VAT | 335,150 | 320,247 | ||||||
| Inventory | 125,962 | 104,355 | ||||||
| Prepaid expenses and other current assets | 21,017 | 58,007 | ||||||
| Security deposit and other long-term assets | 50,012 | 75,843 | ||||||
| Operating lease right-of-use assets, net | 320,123 | 487,797 | ||||||
| Property and equipment, net | 2,567,522 | 3,110,094 | ||||||
| Total Assets | 3,483,992 | 4,322,865 | ||||||
| Notes payable | 918,752 | - | ||||||
| Notes payable - related party | 245,000 | - | ||||||
| Accounts payable | 310,330 | 79,439 | ||||||
| Salary payable | 105,810 | 107,014 | ||||||
| Accrued leasehold improvements liabilities | 315,583 | 1,428 | ||||||
| Accrued liabilities and other payables | 37,432 | 59,329 | ||||||
| Deferred revenue | 88,508 | 2,821 | ||||||
| Deferred grant income | 260,679 | 171,074 | ||||||
| Operating lease obligation | 155,470 | 133,133 | ||||||
| Deferred grant income - noncurrent portion | 351,677 | 495,651 | ||||||
| Operating lease obligation - noncurrent portion | 105,566 | 300,235 | ||||||
| Total Liabilities | 2,894,807 | 1,350,124 | ||||||
| Net Assets | $ | 589,185 | $ | 2,972,741 | ||||
| For the Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Revenues | $ | 1,090,115 | $ | 93,330 | ||||
| Loss from operations | (2,423,451 | ) | (2,985,846 | ) | ||||
| Net loss | $ | (2,442,363 | ) | $ | (2,978,904 | ) | ||
F-21
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 5 – SECURITY DEPOSIT AND OTHER LONG-TERM ASSETS
At December 31, 2020 and 2019, security deposit and other long-term assets consisted of the following:
| December 31, 2020 | December 31, 2019 | |||||||
| Security deposit | $ | 50,012 | $ | 49,993 | ||||
| Prepayment for leasehold improvements | - | 25,850 | ||||||
| Total | $ | 50,012 | $ | 75,843 | ||||
NOTE 6 – PROPERTY AND EQUIPMENT, NET
At December 31, 2020 and 2019, property and equipment consisted of the following:
| December 31, 2020 | December 31, 2019 | |||||||
| Laboratory equipment | $ | 2,268,929 | $ | 2,092,112 | ||||
| Electronic equipment | 234,212 | 216,132 | ||||||
| Tools and furniture | 198,361 | 131,475 | ||||||
| Vehicles | 63,317 | 59,384 | ||||||
| Leasehold improvements | 2,252,449 | 1,784,437 | ||||||
| Software | 26,858 | 25,162 | ||||||
| Total | 5,044,126 | 4,308,702 | ||||||
| Less: accumulated depreciation | (2,476,604 | ) | (1,198,608 | ) | ||||
| Property and equipment, net | $ | 2,567,522 | $ | 3,110,094 | ||||
For the years ended December 31, 2020 and 2019, depreciation expense of property and equipment amounted to $1,147,115 and $889,410, respectively, of which, $28,787 and $0 was included in cost of revenue, $411,986 and $391,602 was included in research and development expenses, and $706,342 and $497,808 was included in general and administrative expense, respectively.
NOTE 7 – NOTES PAYABLE
During 2020, from time to time, the Company acquires loans from various entities to fund its operations. These loans are due within one year and are unsecured and uncollateralized. The Company did not incur any borrowing activity in 2019.
At December 31, 2020, short-term borrowings consisted of the following:
| December 31, 2020 | ||||
| Loan from China Construction Bank, due on February 17, 2021 with annual interest rate of 4.1025%, extended through May 2021 (See Note 15) | $ | 459,376 | ||
| Loan from a third-party company, due on February 8, 2021 with annual interest rate of 4.35%, extended through October 2021 (See Note 15) | 459,376 | |||
| Total | $ | 918,752 | ||
For the year ended December 31, 2020, interest expense related to these borrowings amounted to $12,397 and has been included in interest expense on the accompanying consolidated statements of operations and comprehensive loss.
As of December 31, 2020, the related accrued and unpaid interest for these borrowings was $2,942 and has been included in accrued liabilities and other payables on the accompanying consolidated balance sheets.
F-22
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 8 – RELATED PARTY TRANSACTIONS
Notes Payable - Related Party
During 2020, from time to time, the Company acquired loans from Hebei Senlang Taihe Biological Technology Co. Ltd. (“Taihe”) to fund its operations. These loans are due within one year and are unsecured and uncollateralized. The annual interest rate for these loans is 4.35%. SenlangBio’s largest shareholder is the former executive director of Taihe. The Company did not incur any related party borrowing activity in 2019.
As of December 31, 2020, the outstanding principal amounted to $245,000 and was recorded as “Notes payable – related party” on the accompanying consolidated balance sheets.
For the year ended December 31, 2020, interest expense related to related party borrowings amounted to $11,169 and has been included in interest expense – related party on the accompanying consolidated statements of operations and comprehensive loss. As of December 31, 2020, the related interest for related party borrowings was paid in full.
NOTE 9 – INCOME TAXES
British Virgin Islands
Under the current laws of BVI, Senlang is not subject to tax on income or capital gain. In addition, payments of dividends by the Company to its shareholders are not subject to withholding tax in the BVI.
Hong Kong
Senlang HK is incorporated in Hong Kong and is subject to Hong Kong Profits Tax on the taxable income as reported in its statutory financial statements adjusted in accordance with relevant Hong Kong tax laws. The applicable tax rate is 16.5% on its taxable income generated from operations in Hong Kong. The Company did not make any provisions for Hong Kong profit tax as there were no assessable profits derived from or earned in Hong Kong since inception. Additionally, payments of dividends by the subsidiary incorporated in Hong Kong to the Company are not subject to any Hong Kong withholding tax.
United States
The Company and its subsidiaries have no presence in the United States and does not conduct business in the United States, therefore no United States income tax should be imposed upon the Company and its subsidiaries.
PRC
Senlang BJ, SenlangBio and SenlangBio Clinical Laboratory are subject to PRC Enterprise Income Tax (“EIT”) on the taxable income in accordance with the relevant PRC income tax laws. The EIT rate for companies operating in the PRC is 25%. In the years ended December 2020 and 2019, Senlang BJ, SenlangBio and SenlangBio Clinical Laboratory were each recognized as small low-profit enterprise and received a preferential income tax rate of 10%. The Company did not have any income taxes expense for the years ended December 31, 2020 and 2019 since it incurred losses in the periods. As of December 31, 2020, income tax returns for the tax years ended December 31, 2016 through December 31, 2020 remain open for statutory examination by PRC tax authorities.
Below is a reconciliation of the statutory tax rate to the effective tax rate for the years ended December 31, 2020 and 2019:
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| PRC statutory income tax rate | 25.0 | % | 25.0 | % | ||||
| Effect of income tax exemptions and reliefs | (15.0 | )% | (15.0 | )% | ||||
| Effect of non-deductible expense | 0.1 | % | 0.6 | % | ||||
| Valuation allowance | (10.1 | )% | (10.6 | )% | ||||
| Effective tax rate | 0.0 | % | 0.0 | % | ||||
F-23
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 9 – INCOME TAXES (continued)
PRC (continued)
The Company’s approximate net deferred tax assets as of December 31, 2020 and 2019 were as follows:
| Deferred tax assets: | December 31, 2020 | December 31, 2019 | ||||||
| Net operating loss carryforward | $ | 1,340,709 | $ | 806,933 | ||||
| Valuation allowance | (1,340,709 | ) | (806,933 | ) | ||||
| Net deferred tax assets | $ | - | $ | - | ||||
The Company provided a valuation allowance equal to the deferred income tax assets for the years ended December 31, 2020 and 2019 because it was not known whether future taxable income will be sufficient to utilize the loss carryforward. The potential tax benefit arising from the loss carryforward will begin to expire in 2023.
As of December 31, 2020 and 2019, the Company had no significant uncertain tax positions that qualify for either recognition or disclosure in the financial statements. As of December 31, 2020, income tax returns for the tax years ended December 31, 2016 through December 31, 2020 remain open for statutory examination by PRC tax authorities.
The uncertain tax positions are related to tax years that remain subject to examination by the relevant tax authorities. Based on the outcome of any future examinations, or as a result of the expiration of statute of limitations for specific jurisdictions, it is reasonably possible that the related unrecognized tax benefits for tax positions taken regarding previously filed tax returns, might materially change from those recorded as liabilities for uncertain tax positions in the Company’s consolidated financial statements as of December 31, 2020 and 2019. In addition, the outcome of these examinations may impact the valuation of certain deferred tax assets (such as net operating losses) in future periods. The Company’s policy is to recognize interest and penalties accrued on any unrecognized tax benefits, if any, as a component of income tax expense. The Company does not anticipate any significant increases or decreases to its liability for unrecognized tax benefit within the next twelve months.
According to the PRC Tax Administration and Collection Law, the statute of limitations is three years if the underpayment of income taxes is due to computational errors made by the taxpayer. The statute of limitations will be extended to five years under special circumstances, which are not clearly defined, but an underpayment of income tax liability exceeding RMB100,000 (approximately $15,000) is specifically listed as a special circumstance. In the case of a transfer pricing related adjustment, the statute of limitations is ten years. There is no statute of limitations in the case of tax evasion.
Accounting for Uncertainty in Income Taxes
The tax authority of the PRC government conducts periodic and ad hoc tax filing reviews on business enterprises operating in the PRC after those enterprises complete their relevant tax filings. Therefore, the Company’s tax filings results are subject to change. It is therefore uncertain as to whether the PRC tax authority may take different views about the Company’s tax filings, which may lead to additional tax liabilities.
ASC 740 requires recognition and measurement of uncertain income tax positions using a “more-likely-than-not” approach. The management evaluated the Company’s tax positions and concluded that no provision for uncertainty in income taxes was necessary as of December 31, 2020 and 2019.
F-24
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 10 – EQUITY
The equity structures as of December 31, 2020 was presented after giving retroactive effect to the reorganization of the Company that was completed in April 2021. Immediately before and after the reorganization, the shareholders of SenlangBio controlled Senlang. Therefore, the reorganization is accounted for as a transaction of entities under common control at carrying value and for accounting purpose, the reorganization was accounted for as a recapitalization. The consolidated financial statements are prepared on the basis as if the Reorganization became effective as of the beginning of the first period presented in the accompanying consolidated financial statements of the Company.
Ordinary Shares
On October 15, 2020, Senlang was incorporated in the British Virgin Islands. As of the date of this report, its authorized share capital consists of 50,000 ordinary shares with a par value of $1.00 per share. As of the date of this report, 10,001 ordinary shares were issued and outstanding.
Statutory Reserve
Senlang BJ, SenlangBio and SenlangBio Clinical Laboratory operate in the PRC and are required to reserve 10% of their net profit after income tax, as determined in accordance with the PRC accounting rules and regulations. Appropriation to the statutory reserve by the Company is based on profit arrived at under PRC accounting standards for business enterprises for each year.
The profit arrived at must be set off against any accumulated losses sustained by the Company in prior years, before allocation is made to the statutory reserve. Appropriation to the statutory reserve must be made before distribution of dividends to shareholders. The appropriation is required until the statutory reserve reaches 50% of the registered capital. This statutory reserve is not distributable in the form of cash dividends. Senlang BJ, SenlangBio and SenlangBio Clinical Laboratory did not make any appropriation to statutory reserve during the years ended December 31, 2020 and 2019 as they incurred net losses in the periods. As of December 31, 2020 and 2019, the Company did not have any statutory reserve.
NOTE 11 - CONCENTRATIONS
Customers
The following table sets forth information as to each customer that accounted for 10% or more of the Company’s revenues for the years ended December 31, 2020 and 2019.
| Years Ended December 31, | ||||||||
| Customer | 2020 | 2019 | ||||||
| A | * | 15 | % | |||||
| * | Less than 10% |
One customer, whose outstanding receivable accounted for 10% or more of the Company’s total outstanding accounts receivable at December 31, 2020, accounted for 98.1% of the Company’s total outstanding accounts receivable at December 31, 2020.
One customer, whose outstanding receivable accounted for 10% or more of the Company’s total outstanding accounts receivable at December 31, 2019, accounted for 100.0% of the Company’s total outstanding accounts receivable at December 31, 2019.
Suppliers
The following table sets forth information as to each supplier that accounted for 10% or more of the Company’s purchase for the years ended December 31, 2020 and 2019.
| Years Ended December 31, | ||||||||
| Supplier | 2020 | 2019 | ||||||
| A | 21 | % | * | |||||
| B | 16 | % | 11 | % | ||||
| * | Less than 10% |
Three suppliers, whose outstanding payable accounted for 10% or more of the Company’s total outstanding accounts payable at December 31, 2020, accounted for 77.8% of the Company’s total outstanding accounts payable at December 31, 2020.
Two suppliers, whose outstanding payable accounted for 10% or more of the Company’s total outstanding accounts payable at December 31, 2019, accounted for 61.1% of the Company’s total outstanding accounts payable at December 31, 2019.
F-25
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 12 – SEGMENT INFORMATION
For the years ended December 31, 2020 and 2019, the Company operated in two reportable business segments - (1) general laboratory testing segment, and (2) immunology and hematology testing segment. The Company’s reportable segments are strategic business units that offer different services and products. They are managed separately based on the fundamental differences in their operations. Information with respect to these reportable business segments for the years ended December 31, 2020 and 2019 was as follows:
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Revenues | ||||||||
| General laboratory testing | $ | 649,932 | $ | 77,594 | ||||
| Immunology and hematology testing | 440,183 | 15,736 | ||||||
| Total | 1,090,115 | 93,330 | ||||||
| Cost of revenues | ||||||||
| General laboratory testing | 343,794 | 35,972 | ||||||
| Immunology and hematology testing | 197,444 | 10,269 | ||||||
| Total | 541,238 | 46,241 | ||||||
| Gross profit | ||||||||
| General laboratory testing | 306,138 | 41,622 | ||||||
| Immunology and hematology testing | 242,739 | 5,467 | ||||||
| Total | 548,877 | 47,089 | ||||||
| Operating expenses | ||||||||
| General laboratory testing | 331,209 | 168,380 | ||||||
| Immunology and hematology testing | 2,641,119 | 2,864,555 | ||||||
| Total | 2,972,328 | 3,032,935 | ||||||
| Other (expense) income | ||||||||
| Interest expense | ||||||||
| Immunology and hematology testing | (23,566 | ) | - | |||||
| Total | (23,566 | ) | - | |||||
| Other income | ||||||||
| General laboratory testing | 1,213 | 2,544 | ||||||
| Immunology and hematology testing | 3,441 | 4,398 | ||||||
| Total | 4,654 | 6,942 | ||||||
| Total other (expense) income, net | (18,912 | ) | 6,942 | |||||
| Net loss | ||||||||
| General laboratory testing | 23,858 | 124,214 | ||||||
| Immunology and hematology testing | 2,418,505 | 2,854,690 | ||||||
| Total | 2,442,363 | 2,978,904 | ||||||
| Depreciation | ||||||||
| General laboratory testing | 7,193 | 907 | ||||||
| Immunology and hematology testing | 1,139,922 | 888,503 | ||||||
| Total | 1,147,115 | 889,410 | ||||||
| Capital expenditure | ||||||||
| General laboratory testing | 60,688 | 5,091 | ||||||
| Immunology and hematology testing | 67,421 | 876,597 | ||||||
| Total | $ | 128,109 | $ | 881,688 | ||||
F-26
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 12 – SEGMENT INFORMATION (continued)
| Identifiable long-lived tangible assets at December 31, 2020 and 2019 | December 31, 2020 | December 31, 2019 | ||||||
| General laboratory testing | $ | 62,447 | $ | 14,056 | ||||
| Immunology and hematology testing | 2,505,075 | 3,096,038 | ||||||
| Total | 2,567,522 | 3,110,094 | ||||||
| Total assets at December 31, 2020 and 2019 | ||||||||
| General laboratory testing | 163,560 | 71,234 | ||||||
| Immunology and hematology testing | 3,320,432 | 4,251,631 | ||||||
| Total | $ | 3,483,992 | $ | 4,322,865 | ||||
The Company does not have long-lived assets located outside the PRC.
NOTE 13 – COMMITMENTS AND CONTINCENGIES
Contingencies
From time to time, the Company may be subject to certain legal proceedings, claims and disputes that arise in the ordinary course of business. Although the outcomes of these legal proceedings cannot be predicted, the Company does not believe these actions, in the aggregate, will have a material adverse impact on its financial position, results of operations or liquidity.
Operating Leases Commitment
The Company is a party to leases for office space. Rent expense under all operating leases amounted to approximately $208,000 and $213,000 for the years ended December 31, 2020 and 2019, respectively.
Supplemental cash flow information related to leases for the years ended December 31, 2020 and 2019 is as follows:
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Cash paid for amounts included in the measurement of lease liabilities: | ||||||||
| Operating cash flows paid for operating lease | $ | 209,413 | $ | 217,484 | ||||
| Right-of-use assets obtained in exchange for lease obligation: | ||||||||
| Operating lease | $ | - | $ | - | ||||
The following table summarizes the lease term and discount rate for the Company’s operating lease as of December 31, 2020:
| Operating Lease | ||||
| Weighted average remaining lease term (in years) | 1.27 | |||
| Weighted average discount rate | 4.75 | % | ||
F-27
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 13 – COMMITMENTS AND CONTINCENGIES (continued)
Operating Leases Commitment (continued)
The following table summarizes the maturity of lease liabilities under operating lease as of December 31, 2020:
| For the Year Ending December 31: | Operating Lease | |||
| 2021 | $ | 169,963 | ||
| 2022 | 106,357 | |||
| 2023 and thereafter | - | |||
| Total lease payments | 276,320 | |||
| Amount of lease payments representing interest | (15,284 | ) | ||
| Total present value of operating lease liabilities | $ | 261,036 | ||
| Current portion | $ | 155,470 | ||
| Long-term portion | 105,566 | |||
| Total | $ | 261,036 | ||
Variable Interest Entity Structure
In the opinion of management, (i) the corporate structure of the Company is in compliance with existing PRC laws and regulations; (ii) the Contractual Arrangements are valid and binding, and do not result in any violation of PRC laws or regulations currently in effect; and (iii) the business operations of WFOE, VIE and VIE’s subsidiary are in compliance with existing PRC laws and regulations in all material respects.
However, there are substantial uncertainties regarding the interpretation and application of current and future PRC laws and regulations. Accordingly, the Company cannot be assured that PRC regulatory authorities will not ultimately take a contrary view to the foregoing opinion of its management. If the current corporate structure of the Company or the Contractual Arrangements is found to be in violation of any existing or future PRC laws and regulations, the Company may be required to restructure its corporate structure and operations in the PRC to comply with changing and new PRC laws and regulations. In the opinion of management, the likelihood of loss in respect of the Company’s current corporate structure or the VIE Agreements is remote based on current facts and circumstances.
NOTE 14 – RESTRICTED NET ASSETS
As of December 31, 2020, the Company’s operations are conducted through its PRC subsidiary, VIE and VIE’s subsidiary, which can only pay dividends out of their retained earnings determined in accordance with the accounting standards and regulations in the PRC and after they have met the PRC requirements for appropriation to statutory reserve. In addition, most of the Company’s businesses and assets are denominated in RMB, which is not freely convertible into foreign currencies. All foreign exchange transactions take place either through the People’s Bank of China or other banks authorized to buy and sell foreign currencies at the exchange rates quoted by the People’s Bank of China. Approval of foreign currency payments by the People’s Bank of China or other regulatory institutions requires submitting a payment application form together with suppliers’ invoices, shipping documents and signed contracts. These currency exchange control procedures imposed by the PRC government authorities may restrict the ability of the Company’s PRC subsidiary to transfer their net assets to the Lonlon Biotech Ltd. (the “Parent Company”) through loans, advances or cash dividends.
Schedule I of Article 5-04 of Regulation S-X requires the condensed financial information of the parent company to be filed when the restricted net assets of consolidated subsidiaries exceed 25 percent of consolidated net assets as of the end of the most recently completed fiscal year. For purposes of this test, restricted net assets of consolidated subsidiaries shall mean that amount of the registrant’s proportionate share of net assets of its consolidated subsidiaries (after intercompany eliminations) which as of the end of the most recent fiscal year may not be transferred to the parent company in the form of loans, advances or cash dividends without the consent of a third party. The restricted net assets of the Company’s PRC subsidiary amounted to approximately $589,000 and $2,973,000 as of December 31, 2020 and 2019, respectively.
The Company’s PRC subsidiary’ net assets as of December 31, 2020 and 2019 exceeded 25% of the Company’s consolidated net assets. Accordingly, Parent Company’s condensed financial statements have been prepared in accordance with Rule 5-04 and Rule 12-04 of SEC Regulation S-X, and are as follows.
F-28
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 14 – RESTRICTED NET ASSETS (continued)
Condensed Financial Information of the Parent Company
The Parent Company’s condensed financial statements have been prepared using the same accounting principles and policies described in the notes to the consolidated financial statements, with the only exception being that the Parent Company accounts for its subsidiaries using the equity method. Refer to the consolidated financial statements and notes presented above for additional information and disclosures with respect to these financial statements.
| Parent Company’s Condensed Balance Sheets | ||||||||
| December 31, 2020 |
December 31, 2019 |
|||||||
| ASSETS | ||||||||
| NON-CURRENT ASSETS: | ||||||||
| Investment in subsidiaries | $ | 589,185 | $ | 2,972,741 | ||||
| Total Non-current Assets | 589,185 | 2,972,741 | ||||||
| Total Assets | $ | 589,185 | $ | 2,972,741 | ||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
| Total Liabilities | $ | - | $ | - | ||||
| Commitments and Contingencies | ||||||||
| SHAREHOLDERS’ EQUITY: | ||||||||
|
Ordinary shares, $1.00 par value; 50,000 shares authorized; 10,001 shares issued and outstanding at December 31, 2020 and 2019 * |
10,001 | 10,001 | ||||||
| Additional paid-in capital | 8,946,197 | 8,946,197 | ||||||
| Accumulative deficit | (8,380,014 | ) | (5,937,651 | ) | ||||
| Accumulated other comprehensive income (loss) | 13,001 | (45,806 | ) | |||||
| Total Shareholders’ Equity | 589,185 | 2,972,741 | ||||||
| Total Liabilities and Shareholders’ Equity | $ | 589,185 | $ | 2,972,741 | ||||
| * | The shares amounts are presented on a retroactive basis. |
| Parent Company’s Condensed Statements of Operations and Comprehensive Loss | ||||||||
| For the Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Revenue | $ | - | $ | - | ||||
| Operating expense | - | - | ||||||
| Income attributable to Parent Company only | - | - | ||||||
| Share of loss from investment in subsidiaries | (2,442,363 | ) | (2,978,904 | ) | ||||
| Net loss | (2,442,363 | ) | (2,978,904 | ) | ||||
| Other comprehensive income (loss) | ||||||||
| Unrealized foreign currency translation gain (loss) | 58,807 | (50,204 | ) | |||||
| Comprehensive loss | $ | (2,383,556 | ) | $ | (3,029,108 | ) | ||
F-29
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 14 – RESTRICTED NET ASSETS (continued)
Condensed Financial Information of the Parent Company (continued)
| Parent Company’s Condensed Statements of Cash Flows | ||||||||
| For the Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | (2,442,363 | ) | $ | (2,978,904 | ) | ||
| Adjustments to reconcile net loss to net cash | ||||||||
| provided by operating activities: | ||||||||
| Share of loss from investment in subsidiaries | 2,442,363 | 2,978,904 | ||||||
| NET CASH PROVIDED BY OPERATING ACTIVITIES | - | - | ||||||
| NET INCREASE IN CASH | - | - | ||||||
| CASH - beginning of year | - | - | ||||||
| CASH - end of year | $ | - | $ | - | ||||
Basis of Preparation
The condensed financial information of the Parent Company has been prepared using the same accounting policies as set out in the consolidated financial statements except that the Company used the equity method to account for investment in its subsidiaries.
Certain information and footnote disclosures normally included in financial statements prepared in conformity with accounting principles generally accepted in the United States of America have been condensed or omitted. The Parent Company only financial information has been derived from the Company’s consolidated financial statements and should be read in conjunction with the Company’s consolidated financial statements.
Investment in Subsidiaries
The Company and its subsidiaries were included in the consolidated financial statements where the inter-company balances and transactions were eliminated upon consolidation. For purpose of the Parent Company’s stand-alone financial statements, its investments in subsidiaries were reported using the equity method of accounting. Such investment is presented as “Investment in subsidiaries” on the condensed balance sheets and the subsidiaries’ loss is presented as “Share of loss from investment in subsidiaries” in the condensed statements of operations.
NOTE 15 – SUBSEQUENT EVENTS
In January 2021, the Company renewed its lease agreement to rent office space in Hebei province, China, with a third party (the “Office Lease”). Pursuant to the renewed Office Lease, the daily rent is RMB 382.9 (approximately $59) with a required security deposit of RMB 9,936 (approximately $1,500). The term of the Office Lease is 12 months commencing on January 1, 2021 and expires on December 31, 2021.
In January 2021, the Company borrowed a short-term loan from a third-party company in the principal amount of $459,376. The new loan bears interest rate at 4.35% per annum and is due on October 31, 2021.
In February 2021, the Company signed an extension with China Construction Bank and the borrowing with principal amount of $459,376 was extended through May 17 ,2021. The principal amount was fully repaid on the due date.
In February 2021, the Company signed an extension with a third-party lender and the borrowing with principal amount of $459,376 was extended through October 31, 2021.
In May 2021, the Company borrowed a short-term loan from a related party company in the principal amount of $763,044. The new loan bears interest rate at 4.35% per annum and is due on October 31, 2021.
F-30
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 15 – SUBSEQUENT EVENTS (continued)
On April 26, 2021, Senlang BJ entered into a series of contractual arrangements, or VIE agreements with SenlangBio and 13 equity holders of SenlangBio, through which the Company obtained control and became the primary beneficiary of SenlangBio. As a result, SenlangBio became the Company’s VIE.
On June 13, 2021, the Company entered into a Share Purchase Agreement (the “Purchase Agreement”), by and among the Company, Avalon GloboCare Corp., a Delaware corporation (“Avalon”), the holders of the share capital of Senlang (the “Senlang Shareholders”), the ultimate beneficial owners of the Senlang Shareholders (the “Senlang Beneficial Shareholders” and, together with the Senlang Shareholders, the “Senlang Owners”) and a representative of the Senlang Owners (the “Senlang Representative”). Pursuant to the Purchase Agreement, Avalon will acquire all of the issued and outstanding share capital of the Company in consideration of 81 million shares of the common stock, par value $0.0001 per share, of Avalon (the “Acquisition”).
In connection with the Acquisition, on June 13, 2021, an institutional investor (the “Investor”) entered into an agreement with the related to the purchase of registered capital of the SenlangBio (the “SenlangBio Capital Increase Agreement”) pursuant to which the Investor will acquire an aggregate of up to 13.5% of the equity ownership of the SenlangBio for an aggregate purchase price of RMB 200,000,000 (approximately $30 million) (the “Equity Financing”), which funds will be invested in the SenlangBio in three installments of RMB 67,000,000 (approximately $10 million), RMB 67,000,000 (approximately $10 million) and RMB 66,000,000 (approximately $10 million), the first to be upon the closing of the Acquisition, the second to be within three months after the closing and the third to be within six months after the closing. In addition, pursuant to a Securities Exchange Agreement (the “Exchange Agreement”), by and among the Company, SenlangBio, Avalon and the Investor, dated June 13, 2021, the Investor has the right, exercisable between the six-month and five year-anniversaries of the respective initial closing and installment closings, to elect to exchange, from time to time, all or part of its then-owned equity ownership of SenlangBio for shares (the “Exchange Shares”) of Avalon Common Stock at a fixed exchange price of US$1.21 per share of Avalon Common Stock, which was the market price of the Avalon Common Stock as of the date of the Exchange Agreement under Nasdaq rules. In addition, the Exchange Agreement provides that the Investor may only exchange up to 10% of its total investment amount in any 30-day period.
F-31
LONLON BIOTECH LTD. AND SUBSIDIARIES
CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
March 31, 2021 and 2020
F-32
LONLON BIOTECH LTD. AND SUBSIDIARIES
INDEX TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
March 31, 2021 and 2020
CONTENTS
F-33
LONLON BIOTECH LTD. AND SUBSIDIARIES
CONDENSED CONSOLIDATED BALANCE SHEETS
| March 31, | December 31, | |||||||
| 2021 | 2020 | |||||||
| (Unaudited) | ||||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash | $ | 188,887 | $ | 18,935 | ||||
| Accounts receivable | 636,746 | 45,271 | ||||||
| Recoverable VAT | 343,755 | 335,150 | ||||||
| Inventory | 105,743 | 125,962 | ||||||
| Prepaid expenses and other current assets | 60,949 | 21,017 | ||||||
| Total Current Assets | 1,336,080 | 546,335 | ||||||
| NON-CURRENT ASSETS: | ||||||||
| Security deposit | 49,843 | 50,012 | ||||||
| Operating lease right-of-use assets, net | 266,406 | 320,123 | ||||||
| Property and equipment, net | 2,268,076 | 2,567,522 | ||||||
| Total Non-current Assets | 2,584,325 | 2,937,657 | ||||||
| Total Assets | $ | 3,920,405 | $ | 3,483,992 | ||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Notes payable | $ | 1,373,480 | $ | 918,752 | ||||
| Notes payable - related party | 244,174 | 245,000 | ||||||
| Accounts payable | 564,192 | 310,330 | ||||||
| Salary payable | 102,329 | 105,810 | ||||||
| Accrued leasehold improvements liabilities | 261,106 | 315,583 | ||||||
| Accrued liabilities and other payables | 62,938 | 37,432 | ||||||
| Deferred revenue | 167,201 | 88,508 | ||||||
| Deferred grant income | 183,496 | 260,679 | ||||||
| Operating lease obligation | 151,167 | 155,470 | ||||||
| Total Current Liabilities | 3,110,083 | 2,437,564 | ||||||
| NON-CURRENT LIABILITIES: | ||||||||
| Deferred grant income - noncurrent portion | 304,617 | 351,677 | ||||||
| Operating lease obligation - noncurrent portion | 52,377 | 105,566 | ||||||
| Total Non-current Liabilities | 356,994 | 457,243 | ||||||
| Total Liabilities | 3,467,077 | 2,894,807 | ||||||
| Commitments and Contingencies | ||||||||
| SHAREHOLDERS’ EQUITY: | ||||||||
| Ordinary shares, $1.00 par value; 50,000 shares authorized; 10,001 shares issued and outstanding at March 31, 2021 and December 31, 2020 * | 10,001 | 10,001 | ||||||
| Additional paid-in capital | 8,946,197 | 8,946,197 | ||||||
| Accumulated deficit | (8,515,294 | ) | (8,380,014 | ) | ||||
| Accumulated other comprehensive income | 12,424 | 13,001 | ||||||
| Total shareholders’ equity | 453,328 | 589,185 | ||||||
| Total Liabilities and Shareholders’ Equity | $ | 3,920,405 | $ | 3,483,992 | ||||
| * | The shares amounts are presented on a retroactive basis. |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-34
LONLON BIOTECH LTD. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(Unaudited)
| For the Three Months Ended | ||||||||
| March 31, | ||||||||
| 2021 | 2020 | |||||||
| REVENUES | ||||||||
| General laboratory testing | $ | 945,648 | $ | 42,887 | ||||
| Immunology and hematology testing | 301,857 | - | ||||||
| Total Revenues | 1,247,505 | 42,887 | ||||||
| COST OF REVENUES | ||||||||
| General laboratory testing | 347,911 | 14,117 | ||||||
| Immunology and hematology testing | 89,498 | - | ||||||
| Total Cost of Revenues | 437,409 | 14,117 | ||||||
| GROSS PROFIT | ||||||||
| General laboratory testing | 597,737 | 28,770 | ||||||
| Immunology and hematology testing | 212,359 | - | ||||||
| Total Gross Profit | 810,096 | 28,770 | ||||||
| OPERATING EXPENSES: | ||||||||
| Research and development expenses | 565,331 | 410,893 | ||||||
| General and administrative expenses | 406,188 | 344,252 | ||||||
| Selling and marketing expenses | 52,707 | 8,584 | ||||||
| Grant income | (123,467 | ) | (526,703 | ) | ||||
| Total Operating Expenses | 900,759 | 237,026 | ||||||
| LOSS FROM OPERATIONS | (90,663 | ) | (208,256 | ) | ||||
| OTHER (EXPENSE) INCOME | ||||||||
| Interest expense | (13,647 | ) | - | |||||
| Interest expense - related party | (2,683 | ) | (1,860 | ) | ||||
| Other income | 226 | 1,444 | ||||||
| Total Other Expense, net | (16,104 | ) | (416 | ) | ||||
| LOSS BEFORE INCOME TAXES | (106,767 | ) | (208,672 | ) | ||||
| INCOME TAXES | 28,513 | - | ||||||
| NET LOSS | $ | (135,280 | ) | $ | (208,672 | ) | ||
| COMPREHENSIVE LOSS: | ||||||||
| NET LOSS | $ | (135,280 | ) | $ | (208,672 | ) | ||
| OTHER COMPREHENSIVE LOSS | ||||||||
| Unrealized foreign currency translation loss | (577 | ) | (47,085 | ) | ||||
| COMPREHENSIVE LOSS | $ | (135,857 | ) | $ | (255,757 | ) | ||
| NTE LOSS PER ORDINARY SHARE: | ||||||||
| Basis and diluted * | $ | (13.53) | $ | (20.87) | ||||
| WEIGHTED AVERAGE ORDINARY SHARES OUTSTANDING: | ||||||||
| Basis and diluted * | 10,001 | 10,001 | ||||||
| * | The shares and per share amounts are presented on a retroactive basis. |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-35
LONLON BIOTECH LTD. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN SHAEHOLDERS’ EQUITY
FOR THE THREE MONTHS ENDED MARCH 31, 2021
(Unaudited)
| Accumulated | ||||||||||||||||||||||||
| Ordinary Shares * | Additional | Other | Total | |||||||||||||||||||||
| Number of | Paid-in | Accumulated | Comprehensive | Shareholders’ | ||||||||||||||||||||
| Shares | Amount | Capital | Deficit | Income | Equity | |||||||||||||||||||
| Balance as of December 31, 2020 | 10,001 | $ | 10,001 | $ | 8,946,197 | $ | (8,380,014 | ) | $ | 13,001 | $ | 589,185 | ||||||||||||
| Foreign currency translation adjustment | - | - | - | - | (577 | ) | (577 | ) | ||||||||||||||||
| Net loss for the three months ended March 31, 2021 | - | - | - | (135,280 | ) | - | (135,280 | ) | ||||||||||||||||
| Balance as of March 31, 2021 (unaudited) | 10,001 | $ | 10,001 | $ | 8,946,197 | $ | (8,515,294 | ) | $ | 12,424 | $ | 453,328 | ||||||||||||
| * | The shares amounts are presented on a retroactive basis. |
LONLON BIOTECH LTD. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN SHAEHOLDERS’ EQUITY
FOR THE THREE MONTHS ENDED MARCH 31, 2020
(Unaudited)
| Accumulated | ||||||||||||||||||||||||
| Ordinary Shares* | Other | Total | ||||||||||||||||||||||
| Number of | Paid-in | Accumulated | Comprehensive | Shareholders’ | ||||||||||||||||||||
| Shares | Amount | Capital | Deficit | Loss | Equity | |||||||||||||||||||
| Balance as of December 31, 2019 | 10,001 | $ | 10,001 | $ | 8,946,197 | $ | (5,937,651 | ) | $ | (45,806 | ) | $ | 2,972,741 | |||||||||||
| Foreign currency translation adjustment | - | - | - | - | (47,085 | ) | (47,085 | ) | ||||||||||||||||
| Net loss for the three months ended March 31, 2020 | - | - | - | (208,672 | ) | - | (208,672 | ) | ||||||||||||||||
| Balance as of March 31, 2020 (unaudited) | 10,001 | $ | 10,001 | $ | 8,946,197 | $ | (6,146,323 | ) | $ | (92,891 | ) | $ | 2,716,984 | |||||||||||
| * | The shares amounts are presented on a retroactive basis. |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-36
LONLON BIOTECH LTD. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE TRHEE MONTHS ENDED MARCH 31, 2021 AND 2020
(Unaudited)
| For the Three Months Ended | ||||||||
| March 31, | ||||||||
| 2021 | 2020 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | (135,280 | ) | $ | (208,672 | ) | ||
| Adjustments to reconcile net loss to net cash (used in) provided by operating activities: | ||||||||
| Depreciation | 297,458 | 252,099 | ||||||
| Amortization of right-of-use assets | 53,193 | 44,912 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivable | (597,869 | ) | (1,232 | ) | ||||
| Recoverable VAT | (9,838 | ) | (14,661 | ) | ||||
| Inventory | 20,002 | (9,015 | ) | |||||
| Prepaid expenses and other current assets | (40,425 | ) | 32,917 | |||||
| Security deposit | - | 1,432 | ||||||
| Accounts payable | 257,598 | 35,052 | ||||||
| Salary payable | (3,157 | ) | (10,788 | ) | ||||
| Accrued liabilities and other payables | 25,902 | (22,052 | ) | |||||
| Interest payable - related party | - | 1,860 | ||||||
| Deferred revenue | 79,824 | 135 | ||||||
| Deferred grant income | (123,467 | ) | (42,622 | ) | ||||
| Operating lease obligation | (57,208 | ) | (45,556 | ) | ||||
| NET CASH (USED IN) PROVIDED BY OPERATING ACTIVITIES | (233,267 | ) | 13,809 | |||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Purchase of property and equipment | (57,581 | ) | (18,665 | ) | ||||
| NET CASH USED IN INVESTING ACTIVITIES | (57,581 | ) | (18,665 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Proceeds from borrowings | 462,656 | - | ||||||
| Proceeds from related party’s borrowings | - | 214,829 | ||||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES | 462,656 | 214,829 | ||||||
| EFFECT OF EXCHANGE RATE ON CASH | (1,856 | ) | (5,744 | ) | ||||
| NET INCREASE IN CASH | 169,952 | 204,229 | ||||||
| CASH - beginning of period | 18,935 | 164,994 | ||||||
| CASH - end of period | $ | 188,887 | $ | 369,223 | ||||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | ||||||||
| Cash paid for: | ||||||||
| Interest | $ | 10,292 | $ | - | ||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
F-37
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 1 – ORGANIZATION AND NATURE OF OPERATIONS
Lonlon Biotech Ltd. (“Senlang” or the “Company”) is a holding company incorporated in the British Virgin Islands (“BVI”) on October 15, 2020. The Company is mainly engaged in the business of research and development in relation to CAR-T cell therapy, immune cell therapy and related drug development in the People’s Republic of China (“PRC” or “China”), through a variable interest entity (“VIE” as defined in Note 4), Hebei Senlang Biotechnology Co., Ltd. (“SenlangBio”), which was established on January 29, 2016, and subsidiary of the VIE. SenlangBio also provides immunology and hematology testing services, mainly related to cell therapy, including tumor biomarkers and immunophenotyping. On February 9, 2018, SenlangBio formed a wholly owned subsidiary, Shijiazhuang Senlang Medical Laboratory Co., Ltd. (“SenlangBio Clinical Laboratory”), in China, which focuses on general laboratory testing for patients and other customers, including genomics, proteomics, routine blood/urine testing, COVID-19 PCR/antibody testing etc.
On November 2, 2020, Senlang established a wholly owned subsidiary in Hong Kong, Lonlon Biotech Investment Limited (“Senlang HK”), which is a holding company. On November 20, 2020, Senlang HK established a Wholly Foreign-Owned Enterprise in China, Beijing Langlang Runfeng Biotechnology Co., Ltd. (“Senlang BJ” or “WFOE”).
On April 26, 2021, Senlang BJ entered into a series of contractual arrangements, or VIE agreements with SenlangBio and 13 equity holders of SenlangBio, through which the Company obtained control and became the primary beneficiary of SenlangBio, hereinafter referred to as the Reorganization. As a result, SenlangBio became the Company’s VIE.
On April 26, 2021, the Company completed its reorganization of the entities under the common control of 13 majority shareholders through their 100% controlled entities incorporated in the British Virgin Islands, and indirectly owned a majority of the equity interests of the Company, its subsidiaries, its VIE and the VIE’s subsidiary prior to and after the Reorganization. The Company was established as a holding company of Senlang BJ. Senlang BJ is the primary beneficiary of SenlangBio, and all of these entities are under common control of the Company’s ultimate controlling shareholders before and after the Reorganization, which results in the consolidation of the Company and has been accounted for as a reorganization of entities under common control at carrying value and for accounting purpose, the reorganization was accounted for as a recapitalization. The consolidated financial statements are prepared on the basis as if the Reorganization became effective as of the beginning of the first period presented in the accompanying consolidated financial statements of the Company.
The following chart illustrates the Company’s corporate structure, including its subsidiaries, consolidated variable interest entity and VIE’s subsidiary as of the issuance date of this report:
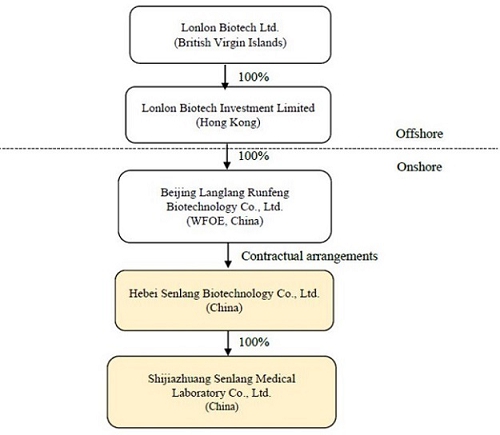
F-38
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 1 – ORGANIZATION AND NATURE OF OPERATIONS (continued)
VIE Agreements with SenlangBio
Upon the completion of the reorganization, the Company, through the WFOE, entered into the following contractual arrangements with the VIE and the VIE’s 13 shareholders that enabled the Company to (1) have power to direct the activities that most significantly affects the economic performance of the VIE, and (2) receive the economic benefits of the VIE that could be significant to the VIE. Accordingly, the WFOE was considered the primary beneficiary of the VIE and had consolidated the VIE and the VIE’s subsidiary’ financial results of operations, assets and liabilities in the Company’s consolidated financial statements.
Contracts that give the Company effective control of the VIE
Equity Pledge Agreement
Under the Equity Pledge Agreement between Senlang BJ, SenlangBio and SenlangBio’s shareholders, SenlangBio’s shareholders agree to pledge all of their equity interests in SenlangBio to Senlang BJ to guarantee the performance of SenlangBio and SenlangBio’s shareholders’ obligations under the Exclusive Technical Consultation and Service Agreement, the Exclusive Purchase Option Agreement, the Shareholder’s Rights Proxy Agreement, and the Spousal Consent (“Transaction Agreements”). Under the terms of the Equity Pledge Agreement, in the event that SenlangBio or SenlangBio’s shareholders breach their respective contractual obligations under the Transaction Agreements or the Equity Pledge Agreement, Senlang BJ, as pledgee, is entitled to directly exercise the pledge right and to notify SenlangBio’s shareholders to immediately repay or pay the loans or other payables under the Transaction Agreements. SenlangBio’s shareholders further agreed not to dispose of the pledged equity interests without prior written consent from Senlang BJ.
The pledge is effective on the date when the registration of the equity pledge is completed with the competent administration for industry and commerce, and the term of validity of the pledge is the same as the longest term of validity in the Transaction Agreements.
The purposes of the Equity Pledge Agreement are to (1) guarantee the performance of SenlangBio and SenlangBio’s shareholders’ obligations under the Transaction Agreements, (2) make sure SenlangBio’s shareholders do not transfer or assign the pledged equity interests, or create or allow any encumbrance that would prejudice interests of Senlang BJ without prior written consent from Senlang BJ, and (3) provide Senlang BJ control over SenlangBio.
In the event SenlangBio or SenlangBio’s shareholders breaches their contractual obligations under the Transaction Agreements or Equity Pledge Agreement, Senlang BJ will be entitled to (1) be compensated on a preferential basis with the proceeds from the conversion, auction or sale of the pledged equity, and (2) notify SenlangBio’s shareholders to immediately repay the loans or other payables under the Transaction Agreements.
Exclusive Purchase Option Agreement
Under the Exclusive Purchase Option Agreement, SenlangBio’s shareholders irrevocably grants Senlang BJ (or its designee) an exclusive right to purchase the equity held by SenlangBio’s shareholders in the SenlangBio in whole or in part at any time during the term of the Agreement; SenlangBio also irrevocably grants Senlang BJ (or its designee) an exclusive right to purchase the assets owned by SenlangBio in whole or in part at any time during the term of the Agreement.
With respect to consideration for equity purchase, Senlang BJ has the right to purchase all or part of the equity held by SenlangBio’s shareholders in SenlangBio at the lowest price permitted by the PRC laws. With respect to the price for asset purchase, Senlang BJ has the right to purchase SenlangBio’s assets at a price equivalent of the net book value of the purchased assets; provided that if the minimum price permitted by the PRC law is higher than the net book value, the minimum price permitted by the PRC laws will prevail.
Under the Exclusive Purchase Option Agreement, Senlang BJ may purchase all or part of the equity held by SenlangBio’s shareholders in SenlangBio and all or part of the assets owned by SenlangBio at any time to the extent permitted by PRC laws. The Exclusive Purchase Option Agreement, together with the Equity Pledge Agreement, Exclusive Technical Consultation and Service Agreement, and the Proxy Agreement, enable Senlang BJ to exercise effective control over SenlangBio. The Exclusive Purchase Option Agreement will become effective upon the affixation of signatures or corporate seals by the Senlang BJ, SenlangBio and SenlangBio’s shareholders, and will remain in effect, unless terminated by Senlang BJ by giving a thirty (30) day advance written notice.
F-39
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 1 – ORGANIZATION AND NATURE OF OPERATIONS (continued)
Contracts that give the Company effective control of the VIE
Shareholder’s Rights Proxy Agreement
Under the Shareholder’s Rights Proxy Agreement, SenlangBio’s shareholders authorize any entity or individual designated by Senlang BJ to act on their behalf as their exclusive agent to exercise all shareholder’s rights under the PRC laws and the Articles of Association, including but not limited to: (a) to convene, attend and vote on all the matters during shareholders’ meetings; (b) to transfer, pledge or dispose of, or create encumbrance on the equity; (c) to receive dividends; (d) to participate in judicial proceedings or execute legal documents in relation to shareholders’ rights; (e) to appoint legal representatives, directors and officers of SenlangBio; and (f) to enter into contracts and exercise the Exclusive Purchase Option Agreement.
The Shareholder’s Rights Proxy Agreement shall remain effective until the earlier of: (a) the date on which SenlangBio’s shareholders are no longer the nominee or actual shareholders of Senlang BJ; (b) the date on which Senlang BJ requests the proxy to be terminated in writing; or (c) the date on which the assets and licenses of SenlangBio have been fully transferred to Senlang BJ.
Contracts that enable the Company to receive substantially all of the economic benefits from the VIE
Exclusive Technical Consultation and Service Agreement
Pursuant to the Exclusive Technical Consultation and Service Agreement between Senlang BJ and SenlangBio, Senlang BJ provides SenlangBio with technical consultation and services, including conducting market research, assisting with developing management and sales plans, implementing relevant technology application, and providing other consultation services for computer network, finance, business, legal affairs, operation, human resources, and other aspects of SenlangBio. Additionally, Senlang BJ agrees to grant SenlangBio its trademarks, software copyrights, management systems, management methods and other intellectual property in relation to its services on a chargeable and revocable basis, but such grant shall not result in the transfer of any intellectual property or create any restriction on Senlang BJ’s full ownership.
For services rendered to SenlangBio under this agreement, Senlang BJ is entitled to collect a service fee calculated based on the complexity of services rendered, time required by Senlang BJ, and the exact contents and commercial value of services rendered. During the term of this agreement, Senlang BJ shall enjoy all the economic benefits derived from SenlangBio’s operation, and in the event of serious difficulties in SenlangBio’s operations, Senlang BJ may provide SenlangBio with financial support, and Senlang BJ has the right to request SenlangBio to cease operation. The Exclusive Technical Consultation and Service Agreement shall remain in effect for ten (10) years and shall be automatically renewed unless it is terminated earlier by Senlang BJ.
Based on the foregoing VIE Agreements, Senlang BJ has effective control of SenlangBio which enables Senlang BJ to receive all of the expected residual returns and absorb the expected losses of the VIE and its subsidiary. Management therefore concludes that the Company, through the above contractual arrangements, has the power to direct the activities that most significantly impact the VIE’s economic performance, bears the risks of and enjoys the rewards normally associated with ownership of the VIE, and therefore the Company is the ultimate primary beneficiary of the VIE. Consequently, the Company consolidates the accounts of SenlangBio and its subsidiary for the periods presented herein, in accordance with Accounting Standards Codification (“ASC”) 810-10, Consolidation. The accompanying consolidated financial statements reflect the activities of Senlang and each of the following entities:
| Name | Background | Ownership | ||
| Subsidiaries: | ||||
| Senlang HK | A Hong Kong company | 100% owned by Senlang | ||
| Incorporated on November 2, 2020 | ||||
| Senlang BJ | A PRC limited liability company and a wholly foreign owned enterprise | 100% owned by Senlang HK | ||
| Incorporated on November 20, 2020 | ||||
| VIE: | ||||
| SenlangBio | A PRC limited liability company | VIE | ||
|
Incorporated on January 29, 2016 Immunology and hematology testing service provider |
||||
| VIE’s subsidiary: | ||||
| SenlangBio | A PRC limited liability company | 100% owned by SenlangBio | ||
| Clinical Laboratory | Incorporated on February 9, 2018 | |||
| General laboratory testing service provider |
F-40
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 2 – BASIS OF PRESENTATION AND GOING CONCERN AND LIQUIDITY
Basis of Presentation
These interim condensed consolidated financial statements of the Company and its subsidiary are unaudited. In the opinion of management, all adjustments (consisting of normal recurring accruals) and disclosures necessary for a fair presentation of these interim condensed consolidated financial statements have been included. The results reported in the condensed consolidated financial statements for any interim periods are not necessarily indicative of the results that may be reported for the entire year. The accompanying condensed consolidated financial statements have been prepared in accordance with the rules and regulations of the Securities and Exchange Commission and do not include all information and footnotes necessary for a complete presentation of financial statements in conformity with accounting principles generally accepted in the United States (“U.S. GAAP”).
The Company’s condensed consolidated financial statements include the accounts of the Company and its subsidiaries, VIE and subsidiary of VIE over which the Company exercises control and, when applicable, entity for which the Company has a controlling financial interest or is the primary beneficiary. All significant intercompany accounts and transactions have been eliminated in consolidation.
Certain information and footnote disclosures normally included in the annual consolidated financial statements prepared in accordance with U.S. GAAP have been condensed or omitted. These condensed consolidated financial statements should be read in conjunction with the Company’s audited consolidated financial statements and notes thereto included in elsewhere in this 8K/A.
Going Concern and Liquidity
The Company currently has limited operations. Currently, the Company’s operations are focused on utilizing cell and gene engineering technologies to generate innovative and transformative cellular immunotherapies for solid and hematologic cancers. The Company provides general laboratory testing and immunology and hematology testing services for patients and other customers in China. These consolidated financial statements have been prepared assuming that the Company will continue as a going concern, which contemplates, among other things, the realization of assets and the satisfaction of liabilities in the normal course of business.
As reflected in the accompanying condensed consolidated financial statements, the Company had a working capital deficit of $1,774,003 at March 31, 2021, and has incurred net loss and negative cash flow from operating activities of $135,280 and $233,267, respectively, for the three months ended March 31, 2021. The Company has a limited operating history and its continued growth is dependent upon the continuation of providing general laboratory testing and immunology and hematology testing services, and obtaining additional financing to fund future obligations and pay liabilities arising from normal business operations. In addition, the current cash balance cannot be projected to cover the operating expenses for the next twelve months from the release date of this report. These matters raise substantial doubt about the Company’s ability to continue as a going concern. The ability of the Company to continue as a going concern is dependent on the Company’s ability to raise additional capital, implement its business plan, and generate significant revenues. There are no assurances that the Company will be successful in its efforts to generate significant revenues, maintain sufficient cash balance or report profitable operations or to continue as a going concern. The Company plans on raising capital through bank and other borrowings to implement its business plan. However, there is no assurance these plans will be realized and that any additional financings will be available to the Company on satisfactory terms and conditions, if any. These condensed consolidated financial statements have been prepared assuming that the Company will continue as a going concern, which contemplates, among other things, the realization of assets and the satisfaction of liabilities in the normal course of business.
The occurrence of an uncontrollable event such as the COVID-19 pandemic could negatively impact the Company’s operations even though the pandemic did not significantly impact the Company’s operation in the first quarter of 2021. However, given the dynamic nature of these circumstances, the uncertainty around the potential resurgence of the COVID-19 cases in China, and the instability of local policies and restrictions, the COVID-19 impact over the Company’s business in the rest of year 2021 cannot be reasonably estimated at this time. If COVID-19 cases resurged in the area the Company conducted its business and local government implemented new restrictions in the effort to contain the spread, it is expected the Company’s business will be negatively impacted.
The accompanying condensed consolidated financial statements do not include any adjustments related to the recoverability or classification of asset-carrying amounts or the amounts and classification of liabilities that may result should the Company be unable to continue as a going concern.
F-41
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Use of Estimates and Assumptions
The preparation of the condensed consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from these estimates. Significant estimates during the three months ended March 31, 2021 and 2020 include the useful lives of property and equipment, assumptions used in assessing impairment of long-term assets, and valuation of deferred tax assets and the associated valuation allowances.
Fair Value of Financial Instruments and Fair Value Measurements
The Company adopted the guidance of Accounting Standards Codification (“ASC”) 820 for fair value measurements which clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
| ● | Level 1-Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date. |
| ● | Level 2-Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other than quoted prices that are observable, and inputs derived from or corroborated by observable market data. |
| ● | Level 3-Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information. |
The carrying values of current assets and current liabilities in the Company’s condensed consolidated balance sheets approximated their fair values as of March 31, 2021 and December 31, 2020 due to their short-term nature.
ASC 825-10 “Financial Instruments”, allows entities to voluntarily choose to measure certain financial assets and liabilities at fair value (fair value option). The fair value option may be elected on an instrument-by-instrument basis and is irrevocable, unless a new election date occurs. If the fair value option is elected for an instrument, unrealized gains and losses for that instrument should be reported in earnings at each subsequent reporting date. The Company did not elect to apply the fair value option to any outstanding instruments.
Cash
Cash consists of cash on hand and cash in the bank. The Company maintains cash with financial institutions in the PRC.
Credit Risk and Uncertainties
The Company’s cash is maintained with state-owned banks within the PRC. Balances at state-owned banks within the PRC are covered by insurance up to RMB 500,000 (approximately $76,000) per bank. Any balance over RMB 500,000 per bank in PRC will not be covered. At March 31, 2021, cash balances held by state-owned banks within the PRC are RMB 1,205,456 (approximately $184,000), of which, RMB 172,460 (approximately $26,000) was not covered by such limited insurance. The Company has not experienced any losses in such accounts and believes it is not exposed to any risks on its cash in bank accounts.
The Company’s operations are carried out in PRC. Accordingly, the Company’s business, financial condition and results of operations may be influenced by the political, economic and legal environment in the PRC, and by the general state of the PRC’s economy. The Company’s operations in PRC are subject to specific considerations and significant risks not typically associated with companies in North America. The Company’s results may be adversely affected by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
Financial instruments which potentially subject the Company to concentrations of credit risk consist principally of trade accounts receivable. A portion of the Company’s sales are credit sales which is to the customer whose ability to pay is dependent upon the industry economics prevailing in Hebei province, China; however, concentrations of credit risk with respect to trade accounts receivable is limited due to short term payment terms. The Company also performs ongoing credit evaluations of its customers to help further reduce credit risk.
F-42
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable are presented net of an allowance for doubtful accounts. The Company maintains allowances for doubtful accounts for estimated losses. The Company reviews the accounts receivable on a periodic basis and makes general and specific allowances when there is doubt as to the collectability of individual balances. In evaluating the collectability of individual receivable balances, the Company considers many factors, including the age of the balance, a customer’s payment history, its current credit-worthiness and current economic trends. Accounts are written off after exhaustive efforts at collection.
Management believes that the accounts receivable are fully collectable. Therefore, no allowance for doubtful accounts is deemed to be required on its accounts receivable at March 31, 2021 and December 31, 2020. The Company historically has not experienced significant uncollectible accounts receivable.
Inventory
Inventory consists of raw materials. Inventory is stated at the lower of cost or net realizable value. Cost is determined using the first-in, first-out (FIFO) method. A reserve is established when management determines that certain inventory may not be saleable. If inventory costs exceed expected net realizable value due to obsolescence or quantities in excess of expected demand, the Company will record reserves for the difference between the cost and the estimated net realizable value. The Company did not record any inventory reserve at March 31, 2021 and December 31, 2020.
Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets primarily consist of prepaid utilities and prepaid service fees, which are recognized as expenses over the related service periods. As of March 31, 2021 and December 31, 2020, prepaid expenses and other current assets amounted to $60,949 and $21,017, respectively.
Property and Equipment
Property and equipment are carried at cost and are depreciated on a straight-line basis over the estimated useful lives of the assets. The estimated useful lives are as follows:
| Useful Life | Estimated Residual Value Rate | |||
| Laboratory equipment | 3 - 5 Years | 5% | ||
| Office equipment and furniture | 3 - 5 Years | 5% | ||
| Vehicles | 4 - 5 Years | 5% | ||
| Leasehold improvements | Shorter of the remaining lease terms or estimated useful life | 0% | ||
| Software | 1 - 2 Years | 0% |
The cost of repairs and maintenance is expensed as incurred; major replacements and improvements are capitalized. When assets are retired or disposed of, the cost and accumulated depreciation are removed from the accounts, and any resulting gains or losses are included in income or loss in the period of disposition. The Company examines impairment of fixed assets when events or changes in circumstances reflect the fact that their recorded value may not be recoverable.
Impairment of Long-lived Assets
In accordance with ASC Topic 360, the Company reviews long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable, or at least annually. The Company recognizes an impairment loss when the sum of expected undiscounted future cash flows is less than the carrying amount of the asset. The amount of impairment is measured as the difference between the asset’s estimated fair value and its book value. There were no triggering events requiring assessment of impairment as of March 31, 2021 and December 31, 2020. For the three months ended March 31, 2021 and 2020, no impairment of long-lived assets was recognized.
F-43
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Value Added Tax
The Company is subject to value added tax (“VAT”) on revenues earned for services provided in the PRC. The amount of VAT liability is determined by applying the applicable tax rates to the invoiced amount of services provided (output VAT) less VAT paid on purchases made with the relevant supporting invoices (input VAT). The Company reports revenue net of PRC’s value added tax for all the periods presented in the consolidated statements of operations and comprehensive loss.
Government Grants and Deferred Grant Income
Government grants related to purchased long-live assets, most of which are laboratory equipment for research and development, are recorded as deferred grant income initially and recognized as grant income on a systematic basis over the useful lives of the assets. Government grants that are to compensate incurred costs, expenses or losses are recognized in the current period. The Company applies the presentation method consistently to the similar government grants in the condensed consolidated financial statements. Government grants that are related to operating activities are included in operating income (loss), otherwise, they are recorded in other income (expense).
Grants and subsidies received from Chinese government are recognized when the proceeds are received or collectible and related milestones have been reached and all contingencies have been resolved. For the three months ended March 31, 2021 and 2020, grant income amounted to $123,467 and $526,703, respectively.
Deferred grant income represents grants collected but not earned as of each of the balance sheet date. This is primarily composed of receipts of the government subsidies for acquisition of lab equipment and reimbursement on office rent and research and development expense. As of March 31, 2021 and December 31, 2020, deferred grant income amounted to $488,113 and $612,356, respectively.
Accrued Liabilities and Other Payables
Accrued liabilities and other payables primarily consist of accrued and unpaid interest related to notes payable, taxes payable, and accrued liabilities for other miscellaneous items. As of March 31, 2021 and December 31, 2020, accrued liabilities and other payables amounted to $62,938 and $37,432, respectively.
Deferred Revenue
Payments received prior to services being performed are recorded as deferred revenue until such time as the services are performed. As of March 31, 2021 and December 31, 2020, deferred revenue amounted to $167,201 and $88,508, respectively.
Revenue Recognition
The Company recognizes revenue under Accounting Standards Codification (“ASC”) Topic 606, Revenue from Contracts with Customers (“ASC 606”). The core principle of the revenue standard is that a company should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. The following five steps are applied to achieve that core principle:
| ● | Step 1: Identify the contract with the customer |
| ● | Step 2: Identify the performance obligations in the contract |
| ● | Step 3: Determine the transaction price |
| ● | Step 4: Allocate the transaction price to the performance obligations in the contract |
| ● | Step 5: Recognize revenue when the company satisfies a performance obligation |
F-44
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Revenue Recognition (continued)
In order to identify the performance obligations in a contract with a customer, a company must assess the promised goods or services in the contract and identify each promised goods or service that is distinct. A performance obligation meets ASC 606’s definition of a “distinct” goods or service (or bundle of goods or services) if both of the following criteria are met:
| ● | The customer can benefit from the goods or service either on its own or together with other resources that are readily available to the customer (i.e., the goods or service is capable of being distinct). |
| ● | The entity’s promise to transfer the goods or service to the customer is separately identifiable from other promises in the contract (i.e., the promise to transfer the goods or service is distinct within the context of the contract). |
If a goods or service is not distinct, the goods or service is combined with other promised goods or services until a bundle of goods or services is identified that is distinct.
The transaction price is the amount of consideration to which an entity expects to be entitled in exchange for transferring promised goods or services to a customer, excluding amounts collected on behalf of third parties (for example, some sales taxes). The consideration promised in a contract with a customer may include fixed amounts, variable amounts, or both. Variable consideration is included in the transaction price only to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. From time to time, the variable consideration may include fees for services performed. The Company uses the expected value method to estimate the amount of variable consideration to be included in the transaction price.
The transaction price is allocated to each performance obligation on a relative standalone selling price basis. The transaction price allocated to each performance obligation is recognized when that performance obligation is satisfied, at a point in time or over time as appropriate.
The Company’s revenues are derived from providing general laboratory testing services and immunology and hematology testing services for patients and other customers. Revenues related to its service offerings are recognized at a point in time when service is rendered. Any payments received in advance of the performance of services are recorded as deferred revenue until such time as the services are performed. Except for deferred revenue, the Company did not have any other contract liability nor contract asset as of March 31, 2021 and December 31, 2020.
The Company does not offer promotional payments, customer coupons, rebates or other cash redemption offers to its customers.
The Company has concluded that its government grants are not within the scope of ASC Topic 606 as they do not meet the definition of a contract with a customer. The Company has concluded that the grants meet the definition of a contribution and are non-reciprocal transactions, and has also concluded that Subtopic 958-605, Not-for-Profit-Entities-Revenue Recognition does not apply, as it is a business entity and the grants are with governmental agencies.
In the absence of applicable guidance under US GAAP, effective January 1, 2018, the Company developed a policy for the recognition of grant revenue when the related costs are incurred or the related long-live assets are purchased, and the right to payment is realized.
The Company believes this policy is consistent with the overarching premise in ASC Topic 606, to ensure that revenue recognition reflects the transfer of promised goods or services to customers in an amount that reflects the consideration that it expects to be entitled to in exchange for those goods or services, even though there is no exchange as defined in ASC Topic 606.
The Company believes the recognition of revenue as costs are incurred or long-live assets are purchased, and amounts become realizable is analogous to the concept of transfer of control of a service over time under ASC Topic 606.
F-45
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Disaggregation of Revenues
In the following table, revenues are disaggregated by segment for the three months ended March 31, 2021 and 2020:
| For the three months ended March 31, | ||||||||||
| Revenue Stream | 2021 | 2020 | Revenue Stream Detail | |||||||
| General laboratory testing | $ | 945,648 | $ | 42,887 | Providing general laboratory testing services, including genomics, proteomics, routine blood/urine testing, COVID-19 PCR/antibody testing etc., to patients and other customers. | |||||
| Immunology and hematology testing | 301,857 | - | Providing immunology and hematology testing services, mainly related to cell therapy, including tumor biomarkers and immunophenotyping, to customers. | |||||||
| Total revenue | $ | 1,247,505 | $ | 42,887 | ||||||
Operating Lease
The Company determines if an arrangement contains a lease at the inception of a contract. Right-of-use assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising from the lease. Right-of- use assets and lease liabilities are recognized at the commencement date based on the present value of the remaining future minimum lease payments. As the interest rate implicit in the Company’s leases is not readily determinable, the Company utilizes its borrowing rates set by the Central Bank of the People’s Republic of China, determined by class of underlying asset, to discount the lease payments.
The Company leases premises for offices under non-cancellable operating leases. Operating lease payments are expensed over the term of lease. The Company leases do not include options to extend nor any restrictions or covenants. The Company has historically been able to renew its office leases. Under the terms of the lease agreements, the Company has no legal or contractual asset retirement obligations at the end of the lease.
Income Taxes
The Company accounts for income taxes using the asset/liability method prescribed by ASC 740, “Income Taxes.” Under this method, deferred tax assets and liabilities are determined based on the difference between the financial reporting and tax bases of assets and liabilities using enacted tax rates that will be in effect in the period in which the differences are expected to reverse. The Company records a valuation allowance to offset deferred tax assets if, based on the weight of available evidence, it is more-likely-than-not that some portion, or all, of the deferred tax assets will not be realized. The effect on deferred taxes of a change in tax rates is recognized as income or loss in the period that includes the enactment date.
The Company follows the accounting guidance for uncertainty in income taxes using the provisions of ASC 740 “Income Taxes”. Using that guidance, tax positions initially need to be recognized in the financial statements when it is more likely than not the position will be sustained upon examination by the tax authorities. As of March 31, 2021 and December 31, 2020, the Company had no significant uncertain tax positions that qualify for either recognition or disclosure in the financial statements. Tax year that remains subject to examination is the years ended December 31, 2017 through December 31, 2020. The Company recognizes interest and penalties related to significant uncertain income tax positions in income tax expense. However, no such interest and penalties were recorded as of March 31, 2021 and December 31, 2020.
F-46
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Loss per share
The Company computes loss per share in accordance with ASC 260, “Earnings per Share” (“ASC 260”). ASC 260 requires companies with complex capital structures to present basic and diluted earnings per share (“EPS”). Basic EPS is measured as net income divided by the weighted average common shares outstanding for the period. Diluted EPS presents the dilutive effect on a per share basis of potential common shares (e.g. convertible securities, options and warrants) as if they had been converted at the beginning of the periods presented, or issuance date, if later. Potential common shares that have an anti-dilutive effect (i.e. those that increase income per share or decrease loss per share) are excluded from the calculation of diluted EPS. As of March 31, 2021 and 2020, there were no dilutive shares.
Foreign Currency Translation
The reporting currency of the Company is U.S. Dollars. The functional currency of the parent company, Senlang, and Senlang HK, is the U.S. dollar and the functional currency of Senlang BJ, SenlangBio, and SenlangBio Clinical Laboratory is the Chinese Renminbi (“RMB”).
Monetary assets and liabilities denominated in currencies other than the reporting currency are translated into the reporting currency at the rates of exchange prevailing at the balance sheet date. Revenue and expenses are translated using average rates during each reporting period, and shareholders’ equity is translated at historical exchange rates. Cash flows are also translated at average translation rates for the periods, therefore, amounts reported on the statement of cash flows will not necessarily agree with changes in the corresponding balances on the consolidated balance sheet. Translation adjustments resulting from the process of translating the local currency financial statements into U.S. dollars are included in determining comprehensive income/loss. Transactions denominated in foreign currencies are translated into the functional currency at the exchange rates prevailing on the transaction dates. Assets and liabilities denominated in foreign currencies are translated into the functional currency at the exchange rates prevailing at the balance sheet date with any transaction gains and losses that arise from exchange rate fluctuations on transactions denominated in a currency other than the functional currency are included in the results of operations as incurred.
All of the Company’s revenue transactions are transacted in the functional currency of the Company. The Company does not enter into any material transaction in foreign currencies. Transaction gains or losses have not had, and are not expected to have, a material effect on the results of operations of the Company.
Asset and liability accounts at March 31, 2021 and December 31, 2020 were translated at 6.5527 RMB and 6.5306 RMB to $1.00, respectively, which were the exchange rates on the balance sheet dates. Equity accounts were stated at their historical rates. The average translation rates applied to the statements of operations for the three months ended March 31, 2021 and 2020 were 6.4843 RMB and 6.9823 RMB to $1.00, respectively. Cash flows from the Company’s operations are calculated based upon the local currencies using the average translation rate.
Commitment and Contingencies
In the normal course of business, the Company is subject to contingencies, such as legal proceedings and claims arising out of its business, that cover a wide range of matters. Liabilities for such contingencies are recorded when it is probable that a liability has been incurred and the amount of the assessment can be reasonably estimated.
Segment Reporting
The Company uses “the management approach” in determining reportable operating segments. The management approach considers the internal organization and reporting used by the Company’s chief operating decision maker for making operating decisions and assessing performance as the source for determining the Company’s reportable segments. The Company’s chief operating decision maker is its Chief Executive Officer (“CEO”), who reviews operating results to make decisions about allocating resources and assessing performance for the entire Company. The Company has determined that it has two reportable business segments: general laboratory testing segment, and immunology and hematology testing segment. These reportable segments offer different types of services and products, have different types of revenue, and are managed separately as each requires different operating strategies and management expertise.
Related Parties
Parties are considered to be related to the Company if the parties, directly or indirectly, through one or more intermediaries, control, are controlled by, or are under common control with the Company. Related parties also include principal owners of the Company, its management, members of the immediate families of principal owners of the Company and its management and other parties with which the Company may deal with if one party controls or can significantly influence the management or operating policies of the other to an extent that one of the transacting parties might be prevented from fully pursuing its own separate interests. The Company discloses all significant related party transactions.
F-47
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Recent Accounting Standards
In August 2018, the FASB issued ASU No. 2018-13, Fair Value Measurement (Topic 820): Disclosure Framework-Changes to the Disclosure Requirements for Fair Value Measurement. The objective of ASU 2018-13 is to improve the effectiveness of disclosures in the notes to the financial statements by removing, modifying, and adding certain fair value disclosure requirements to facilitate clear communication of the information required by generally accepted accounting principles. The amendments are effective for all entities for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2019 with early adoption permitted upon issuance of this ASU. The adoption of ASU 2018 – 13 did not have a material impact on the Company’s condensed consolidated financial statements.
In June 2016, the FASB issued ASU 2016-13, Financial Instruments - Credit Losses (“Topic 326”). The ASU introduces a new accounting model, the Current Expected Credit Losses model (“CECL”), which requires earlier recognition of credit losses and additional disclosures related to credit risk. The CECL model utilizes a lifetime expected credit loss measurement objective for the recognition of credit losses at the time the financial asset is originated or acquired. ASU 2016-13 is effective for annual period beginning after December 15, 2022, including interim reporting periods within those annual reporting periods. The Company expects that the adoption will not have a material impact on the Company’s condensed consolidated financial statements.
In December 2019, the FASB issued ASU 2019-12, Simplifying the Accounting for Income Taxes, as part of its Simplification Initiative to reduce the cost and complexity in accounting for income taxes. This standard removes certain exceptions related to the approach for intra period tax allocation, the methodology for calculating income taxes in an interim period and the recognition of deferred tax liabilities for outside basis differences. It also amends other aspects of the guidance to help simplify and promote consistent application of GAAP. The guidance is effective for interim and annual periods beginning after December 15, 2020, with early adoption permitted. The adoption of ASU 2019-12 did not have a material impact on the Company’s consolidated financial statements.
Other accounting standards that have been issued or proposed by FASB that do not require adoption until a future date are not expected to have a material impact on the consolidated financial statements upon adoption. The Company does not discuss recent pronouncements that are not anticipated to have an impact on or are unrelated to its consolidated financial condition, results of operations, cash flows or disclosures.
NOTE 4 – VARIABLE INTEREST ENTITY AND OTHER CONSOLIDATION MATTERS
On April 26, 2021, Senlang BJ entered into VIE Agreements with SenlangBio and 13 shareholders of SenlangBio. The key terms of these VIE Agreements are summarized in “NOTE 1 – ORGANIZATION AND NATURE OF OPERATIONS” above. As a result of the VIE Agreements, the Company classifies SenlangBio as a VIE.
A VIE is an entity that has either a total equity investment that is insufficient to permit the entity to finance its activities without additional subordinated financial support, or whose equity investors lack the characteristics of a controlling financial interest, such as through voting rights, right to receive the expected residual returns of the entity or obligation to absorb the expected losses of the entity. The variable interest holder, if any, that has a controlling financial interest in a VIE is deemed to be the primary beneficiary and must consolidate the VIE. Senlang BJ is deemed to have a controlling financial interest and be the primary beneficiary of SenlangBio, because it has both of the following characteristics:
| 1. | Power to direct activities of a VIE that most significantly impact the entity’s economic performance, and |
| 2. | Obligation to absorb losses of the entity that could potentially be significant to the VIE or right to receive benefits from the entity that could potentially be significant to the VIE. |
Pursuant to the VIE Agreements, SenlangBio pays service fees equal to all of its net income to Senlang BJ. At the same time, Senlang BJ is entitled to receive all of SenlangBio’s expected residual returns. The VIE Agreements are designed so that SenlangBio operates for the benefit of the Company. Accordingly, the accounts of SenlangBio are consolidated in the accompanying financial statements pursuant to ASC 810-10, Consolidation. In addition, its financial positions and results of operations are included in the Company’s consolidated financial statements.
F-48
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 4 – VARIABLE INTEREST ENTITY AND OTHER CONSOLIDATION MATTERS (continued)
In addition, as all of these VIE agreements are governed by PRC law and provide for the resolution of disputes through arbitration in the PRC, they would be interpreted in accordance with PRC law and any disputes would be resolved in accordance with PRC legal procedures. The legal environment in the PRC is not as developed as in other jurisdictions, such as the United States. As a result, uncertainties in the PRC legal system could further limit the Company’s ability to enforce these VIE agreements. Furthermore, these contracts may not be enforceable in China if PRC government authorities or courts take a view that such contracts contravene PRC laws and regulations or are otherwise not enforceable for public policy reasons. In the event the Company is unable to enforce these VIE agreements, it may not be able to exert effective control over SenlangBio and its ability to conduct its business may be materially and adversely affected.
All of the Company’s main current operations are conducted through SenlangBio and subsidiary of SenlangBio. Current regulations in China permit SenlangBio to pay dividends to the Company only out of its accumulated distributable profits, if any, determined in accordance with its article of association and PRC accounting standards and regulations. The ability of SenlangBio to make dividends and other payments to the Company may be restricted by factors including changes in applicable foreign exchange and other laws and regulations.
The following consolidated financial information of the VIE and VIE’s subsidiary as a whole as of March 31, 2021 and December 31, 2020 and for the three months ended March 31, 2021 and 2020 was included in the accompanying condensed consolidated financial statements of the Company. Transactions between the VIE and VIE’s subsidiary are eliminated in the financial information presented below:
| March 31, 2021 | December 31, 2020 | |||||||
| Cash | $ | 188,887 | $ | 18,935 | ||||
| Accounts receivable | 636,746 | 45,271 | ||||||
| Recoverable VAT | 343,755 | 335,150 | ||||||
| Inventory | 105,743 | 125,962 | ||||||
| Prepaid expenses and other current assets | 60,949 | 21,017 | ||||||
| Security deposit | 49,843 | 50,012 | ||||||
| Operating lease right-of-use assets, net | 266,406 | 320,123 | ||||||
| Property and equipment, net | 2,268,076 | 2,567,522 | ||||||
| Total Assets | 3,920,405 | 3,483,992 | ||||||
| Notes payable | 1,373,480 | 918,752 | ||||||
| Notes payable - related party | 244,174 | 245,000 | ||||||
| Accounts payable | 564,192 | 310,330 | ||||||
| Salary payable | 102,329 | 105,810 | ||||||
| Accrued leasehold improvements liabilities | 261,106 | 315,583 | ||||||
| Accrued liabilities and other payables | 62,938 | 37,432 | ||||||
| Deferred revenue | 167,201 | 88,508 | ||||||
| Deferred grant income | 183,496 | 260,679 | ||||||
| Operating lease obligation | 151,167 | 155,470 | ||||||
| Deferred grant income - noncurrent portion | 304,617 | 351,677 | ||||||
| Operating lease obligation - noncurrent portion | 52,377 | 105,566 | ||||||
| Total Liabilities | 3,467,077 | 2,894,807 | ||||||
| Total shareholders’ equity | $ | 453,328 | $ | 589,185 | ||||
F-49
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 4 – VARIABLE INTEREST ENTITY AND OTHER CONSOLIDATION MATTERS (continued)
| For the Three Months Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| Revenues | $ | 1,247,505 | $ | 42,887 | ||||
| Loss from operations | (90,663 | ) | (208,256 | ) | ||||
| Net loss | $ | (135,280 | ) | $ | (208,672 | ) | ||
NOTE 5 – PROPERTY AND EQUIPMENT, NET
At March 31, 2021 and December 31, 2020, property and equipment consisted of the following:
| March 31, 2021 | December 31, 2020 | |||||||
| Laboratory equipment | $ | 2,261,276 | $ | 2,268,929 | ||||
| Electronic equipment | 235,628 | 234,212 | ||||||
| Tools and furniture | 199,053 | 198,361 | ||||||
| Vehicles | 63,104 | 63,317 | ||||||
| Leasehold improvements | 2,244,852 | 2,252,449 | ||||||
| Software | 26,737 | 26,858 | ||||||
| Total | 5,030,650 | 5,044,126 | ||||||
| Less: accumulated depreciation | (2,762,574 | ) | (2,476,604 | ) | ||||
| Property and equipment, net | $ | 2,268,076 | $ | 2,567,522 | ||||
For the three months ended March 31, 2021 and 2020, depreciation expense of property and equipment amounted to $297,458 and $252,099, respectively, of which, $14,586 and $0 was included in cost of revenue, $96,998 and $107,137 was included in research and development expenses, and $185,874 and $144,962 was included in general and administrative expense, respectively.
NOTE 6 – NOTES PAYABLE
From time to time, the Company acquires loans from various entities to fund its operations. These loans are due within one year and are unsecured and uncollateralized. At March 31, 2021 and December 31, 2020, short-term borrowings consisted of the following:
| March 31, 2021 | December 31, 2020 | |||||||
| Loan from China Construction Bank, due on February 17, 2021 with annual interest rate of 4.1025%, extended to May 17, 2021 with annual interest rate of 3.8525% and repaid on the extended date (See Note 14) | $ | 457,827 | $ | 459,376 | ||||
| Loan from a third-party company, due on February 8, 2021 with annual interest rate of 4.35%, extended through October 2021 | 457,827 | 459,376 | ||||||
| Loan from a third-party company, due on October 31, 2021 with annual interest rate of 4.35% | 457,826 | - | ||||||
| Total | $ | 1,373,480 | $ | 918,752 | ||||
For the three months ended March 31, 2021, interest expense related to borrowings amounted to $13,647 and have been included in interest expense on the accompanying unaudited condensed consolidated statements of operations and comprehensive loss. The Company had neither borrowing activity nor interest expense in the first quarter of 2020.
As of March 31, 2021 and December 31, 2020, the related accrued and unpaid interest for borrowings was $8,907 and $2,942, respectively, and have been included in accrued liabilities and other payables on the accompanying condensed consolidated balance sheets.
F-50
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 7 – RELATED PARTY TRANSACTIONS
Notes Payable - Related Party
From time to time, the Company acquires loans from Hebei Senlang Taihe Biological Technology Co. Ltd. (“Taihe”) to fund its operations. These loans are due within one year and are unsecured and uncollateralized. The annual interest rate for these loans is 4.35%. SenlangBio’s largest shareholder is the former executive director and currently holds 80% of equity interest of Taihe.
As of March 31, 2021 and December 31, 2020, the outstanding principal amounted to $244,174 and $245,000, respectively, and were recorded as “Notes payable – related party” on the accompanying condensed consolidated balance sheets.
For the three months ended March 31, 2021 and 2020, interest expense related to related party borrowings amounted to $2,683 and $1,860, respectively, and have been included in interest expense – related party on the accompanying condensed consolidated statements of operations and comprehensive loss. As of both March 31, 2021 and December 31, 2020, the related interest for related party borrowings was paid in full.
NOTE 8 – INCOME TAXES
British Virgin Islands
Under the current laws of BVI, Senlang is not subject to tax on income or capital gain. In addition, payments of dividends by the Company to its shareholders are not subject to withholding tax in the BVI.
Hong Kong
Senlang HK is incorporated in Hong Kong and is subject to Hong Kong Profits Tax on the taxable income as reported in its statutory financial statements adjusted in accordance with relevant Hong Kong tax laws. The applicable tax rate is 16.5% on its taxable income generated from operations in Hong Kong. The Company did not make any provisions for Hong Kong profit tax as there were no assessable profits derived from or earned in Hong Kong since inception. Additionally, payments of dividends by the subsidiary incorporated in Hong Kong to the Company are not subject to any Hong Kong withholding tax.
United States
The Company and its subsidiaries have no presence in the United States and does not conduct business in the United States, therefore no United States income tax should be imposed upon the Company and its subsidiaries.
PRC
Senlang BJ, SenlangBio and SenlangBio Clinical Laboratory are subject to PRC Enterprise Income Tax (“EIT”) on the taxable income in accordance with the relevant PRC income tax laws. The EIT rate for companies operating in the PRC is 25%. In the year ended December 2020, Senlang BJ, SenlangBio and SenlangBio Clinical Laboratory were each recognized as small low-profit enterprise and received a preferential income tax rate of 10%. In the year ending December 2021, Senlang BJ, SenlangBio and SenlangBio Clinical Laboratory are each expected to be recognized as small low-profit enterprise and are expected to receive a preferential income tax rate of 10%. The Company did not have any income taxes expense for the years ended December 31, 2020 since it incurred losses in the periods. As of March 31, 2021, income tax returns for the tax years ended December 31, 2017 through December 31, 2020 remain open for statutory examination by PRC tax authorities.
Below is a reconciliation of the statutory tax rate to the effective tax rate for the three months ended March 31, 2021 and 2020:
| For the three months ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| PRC statutory income tax rate | 25.0 | % | 25.0 | % | ||||
| Effect of income tax exemptions and reliefs | (15.0 | )% | (15.0 | )% | ||||
| Effect of non-deductible expense | 0.1 | % | 0.1 | % | ||||
| Valuation allowance | (36.8 | )% | (10.1 | )% | ||||
| Effective tax rate | (26.7 | )% | 0.0 | % | ||||
The Company’s approximate net deferred tax assets as of March 31, 2021 and December 31, 2020 were as follows:
| Deferred tax assets: | March 31, 2021 | December 31, 2020 | ||||||
| Net operating loss carryforward | $ | 1,426,050 | $ | 1,340,709 | ||||
| Valuation allowance | (1,426,050 | ) | (1,340,709 | ) | ||||
| Net deferred tax assets | $ | - | $ | - | ||||
F-51
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 8 – INCOME TAXES (continued)
PRC (continued)
The Company provided a valuation allowance equal to the deferred income tax assets as of March 31, 2021 and December 31, 2020 because it was not known whether future taxable income will be sufficient to utilize the loss carryforward. The potential tax benefit arising from the loss carryforward will begin to expire in 2023.
As of March 31, 2021 and December 31, 2020, the Company had no significant uncertain tax positions that qualify for either recognition or disclosure in the financial statements. As of March 31, 2021, income tax returns for the tax years ended December 31, 2017 through December 31, 2020 remain open for statutory examination by PRC tax authorities.
The uncertain tax positions are related to tax years that remain subject to examination by the relevant tax authorities. Based on the outcome of any future examinations, or as a result of the expiration of statute of limitations for specific jurisdictions, it is reasonably possible that the related unrecognized tax benefits for tax positions taken regarding previously filed tax returns, might materially change from those recorded as liabilities for uncertain tax positions in the Company’s consolidated financial statements as of March 31, 2021 and December 31, 2020. In addition, the outcome of these examinations may impact the valuation of certain deferred tax assets (such as net operating losses) in future periods. The Company’s policy is to recognize interest and penalties accrued on any unrecognized tax benefits, if any, as a component of income tax expense. The Company does not anticipate any significant increases or decreases to its liability for unrecognized tax benefit within the next twelve months.
According to the PRC Tax Administration and Collection Law, the statute of limitations is three years if the underpayment of income taxes is due to computational errors made by the taxpayer. The statute of limitations will be extended to five years under special circumstances, which are not clearly defined, but an underpayment of income tax liability exceeding RMB100,000 (approximately $15,000) is specifically listed as a special circumstance. In the case of a transfer pricing related adjustment, the statute of limitations is ten years. There is no statute of limitations in the case of tax evasion.
Accounting for Uncertainty in Income Taxes
The tax authority of the PRC government conducts periodic and ad hoc tax filing reviews on business enterprises operating in the PRC after those enterprises complete their relevant tax filings. Therefore, the Company’s tax filings results are subject to change. It is therefore uncertain as to whether the PRC tax authority may take different views about the Company’s tax filings, which may lead to additional tax liabilities.
ASC 740 requires recognition and measurement of uncertain income tax positions using a “more-likely-than-not” approach. The management evaluated the Company’s tax positions and concluded that no provision for uncertainty in income taxes was necessary as of December 31, 2020 and 2019.
NOTE 9 – EQUITY
The equity structures as of March 31, 2021 was presented after giving retroactive effect to the reorganization of the Company that was completed in April 2021. Immediately before and after the reorganization, the shareholders of SenlangBio controlled Senlang. Therefore, the reorganization is accounted for as a transaction of entities under common control at carrying value and for accounting purpose, the reorganization was accounted for as a recapitalization. The consolidated financial statements are prepared on the basis as if the Reorganization became effective as of the beginning of the first period presented in the accompanying consolidated financial statements of the Company.
Ordinary Shares
On October 15, 2020, Senlang was incorporated in the British Virgin Islands. As of the date of this report, its authorized share capital consists of 50,000 ordinary shares with a par value of $1.00 per share. As of the date of this report, 10,001 ordinary shares were issued and outstanding.
Statutory Reserve
Senlang BJ, SenlangBio and SenlangBio Clinical Laboratory operate in the PRC and are required to reserve 10% of their net profit after income tax, as determined in accordance with the PRC accounting rules and regulations. Appropriation to the statutory reserve by the Company is based on profit arrived at under PRC accounting standards for business enterprises for each year.
The profit arrived at must be set off against any accumulated losses sustained by the Company in prior years, before allocation is made to the statutory reserve. Appropriation to the statutory reserve must be made before distribution of dividends to shareholders. The appropriation is required until the statutory reserve reaches 50% of the registered capital. This statutory reserve is not distributable in the form of cash dividends. Senlang BJ, SenlangBio and SenlangBio Clinical Laboratory did not make any appropriation to statutory reserve during the three months ended March 31, 2021 and 2020. As of both March 31, 2021 and December 31, 2020, the Company did not have any statutory reserve.
F-52
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 10 - CONCENTRATIONS
Customers
The following table sets forth information as to each customer that accounted for 10% or more of the Company’s revenues for the three months ended March 31, 2021 and 2020.
| Three Months Ended March 31, | ||||||||
| Customer | 2021 | 2020 | ||||||
| A | 43 | % | * | |||||
| B | 13 | % | * | |||||
| C | * | 20 | % | |||||
| D | * | 10 | % | |||||
| E | * | 10 | % | |||||
| * | Less than 10% |
Two customers, whose outstanding receivable accounted for 10% or more of the Company’s total outstanding accounts receivable at March 31, 2021, accounted for 97% of the Company’s total outstanding accounts receivable at March 31, 2021.
One customer, whose outstanding receivable accounted for 10% or more of the Company’s total outstanding accounts receivable at December 31, 2020, accounted for 98.1% of the Company’s total outstanding accounts receivable at December 31, 2020.
Suppliers
The following table sets forth information as to each supplier that accounted for 10% or more of the Company’s purchase for the three months ended March 31, 2021 and 2020.
| Three Months Ended March 31, | ||||||||
| Supplier | 2021 | 2020 | ||||||
| A | 40 | % | * | |||||
| B | 19 | % | * | |||||
| C | * | 20 | % | |||||
| D | * | 10 | % | |||||
| * | Less than 10% |
Two suppliers, whose outstanding payable accounted for 10% or more of the Company’s total outstanding accounts payable at March 31, 2021, accounted for 35.7% of the Company’s total outstanding accounts payable at March 31, 2021.
Three suppliers, whose outstanding payable accounted for 10% or more of the Company’s total outstanding accounts payable at December 31, 2020, accounted for 77.8% of the Company’s total outstanding accounts payable at December 31, 2020.
NOTE 11 – SEGMENT INFORMATION
For the three months ended March 31, 2021 and 2020, the Company operated in two reportable business segments - (1) general laboratory testing segment, and (2) immunology and hematology testing segment. The Company’s reportable segments are strategic business units that offer different services and products. They are managed separately based on the fundamental differences in their operations. Information with respect to these reportable business segments for the three months ended March 31, 2021 and 2020 was as follows:
F-53
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 11 – SEGMENT INFORMATION (continued)
| Three Months Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| Revenues | ||||||||
| General laboratory testing | $ | 945,648 | $ | 42,887 | ||||
| Immunology and hematology testing | 301,857 | - | ||||||
| Total | 1,247,505 | 42,887 | ||||||
| Cost of revenues | ||||||||
| General laboratory testing | 347,911 | 14,117 | ||||||
| Immunology and hematology testing | 89,498 | - | ||||||
| Total | 437,409 | 14,117 | ||||||
| Gross profit | ||||||||
| General laboratory testing | 597,737 | 28,770 | ||||||
| Immunology and hematology testing | 212,359 | - | ||||||
| Total | 810,096 | 28,770 | ||||||
| Operating expenses | ||||||||
| General laboratory testing | 59,480 | 50,969 | ||||||
| Immunology and hematology testing | 841,279 | 186,057 | ||||||
| Total | 900,759 | 237,026 | ||||||
| Other (expense) income | ||||||||
| Interest expense | ||||||||
| Immunology and hematology testing | (16,330 | ) | (1,860 | ) | ||||
| Other income | ||||||||
| General laboratory testing | 96 | 1,072 | ||||||
| Immunology and hematology testing | 130 | 372 | ||||||
| Total | 226 | 1,444 | ||||||
| Total other expense, net | (16,104 | ) | (416 | ) | ||||
| Income taxes | ||||||||
| General laboratory testing | 28,513 | - | ||||||
| Immunology and hematology testing | - | - | ||||||
| Total | 28,513 | - | ||||||
| Net income (loss) | ||||||||
| General laboratory testing | 509,840 | (21,127 | ) | |||||
| Immunology and hematology testing | (645,120 | ) | (187,545 | ) | ||||
| Total | (135,280 | ) | (208,672 | ) | ||||
| Depreciation | ||||||||
| General laboratory testing | 3,970 | 3,456 | ||||||
| Immunology and hematology testing | 293,488 | 248,643 | ||||||
| Total | 297,458 | 252,099 | ||||||
| Capital expenditure | ||||||||
| General laboratory testing | 1,376 | 6,874 | ||||||
| Immunology and hematology testing | 56,205 | 11,791 | ||||||
| Total | $ | 57,581 | $ | 18,665 | ||||
F-54
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 11 – SEGMENT INFORMATION (continued)
| Identifiable long-lived tangible assets at March 31, 2021 and December 31, 2020 | March 31, 2021 | December 31, 2020 | ||||||
| General laboratory testing | $ | 59,669 | $ | 62,447 | ||||
| Immunology and hematology testing | 2,208,407 | 2,505,075 | ||||||
| Total | 2,268,076 | 2,567,522 | ||||||
| Total assets at March 31, 2021 and December 31, 2020 | ||||||||
| General laboratory testing | 809,399 | 163,560 | ||||||
| Immunology and hematology testing | 3,111,006 | 3,320,432 | ||||||
| Total | $ | 3,920,405 | $ | 3,483,992 | ||||
The Company does not have long-lived assets located outside the PRC.
NOTE 12 – COMMITMENTS AND CONTINCENGIES
Contingencies
From time to time, the Company may be subject to certain legal proceedings, claims and disputes that arise in the ordinary course of business. Although the outcomes of these legal proceedings cannot be predicted, the Company does not believe these actions, in the aggregate, will have a material adverse impact on its financial position, results of operations or liquidity.
Operating Leases Commitment
The Company is a party to leases for office space. Rent expense under all operating leases amounted to approximately $55,000 and $54,000 for the three months ended March 31, 2021 and 2020, respectively.
Supplemental cash flow information related to leases for the three months ended March 31, 2021 and 2020 is as follows:
| Three Months Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| Cash paid for amounts included in the measurement of lease liabilities: | ||||||||
| Operating cash flows paid for operating lease | $ | 59,039 | $ | 54,687 | ||||
| Right-of-use assets obtained in exchange for lease obligation: | ||||||||
| Operating lease | $ | - | $ | - | ||||
The following table summarizes the lease term and discount rate for the Company’s operating lease as of March 31, 2021:
| Operating Lease | ||||
| Weighted average remaining lease term (in years) | 1.02 | |||
| Weighted average discount rate | 4.75 | % | ||
F-55
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 12 – COMMITMENTS AND CONTINCENGIES (continued)
Operating Leases Commitment (continued)
The following table summarizes the maturity of lease liabilities under operating lease as of March 31, 2021:
| For the Twelve-month Period Ending March 31: | Operating Lease | |||
| 2022 | $ | 162,681 | ||
| 2023 | 52,999 | |||
| 2024 and thereafter | - | |||
| Total lease payments | 215,680 | |||
| Amount of lease payments representing interest | (12,136 | ) | ||
| Total present value of operating lease liabilities | $ | 203,544 | ||
| Current portion | $ | 151,167 | ||
| Long-term portion | 52,377 | |||
| Total | $ | 203,544 | ||
Variable Interest Entity Structure
In the opinion of management, (i) the corporate structure of the Company is in compliance with existing PRC laws and regulations; (ii) the Contractual Arrangements are valid and binding, and do not result in any violation of PRC laws or regulations currently in effect; and (iii) the business operations of WFOE, VIE and VIE’s subsidiary are in compliance with existing PRC laws and regulations in all material respects.
However, there are substantial uncertainties regarding the interpretation and application of current and future PRC laws and regulations. Accordingly, the Company cannot be assured that PRC regulatory authorities will not ultimately take a contrary view to the foregoing opinion of its management. If the current corporate structure of the Company or the Contractual Arrangements is found to be in violation of any existing or future PRC laws and regulations, the Company may be required to restructure its corporate structure and operations in the PRC to comply with changing and new PRC laws and regulations. In the opinion of management, the likelihood of loss in respect of the Company’s current corporate structure or the VIE Agreements is remote based on current facts and circumstances.
NOTE 13 – RESTRICTED NET ASSETS
As of March 31, 2021, the Company’s operations are conducted through its PRC subsidiary, VIE and VIE’s subsidiary, which can only pay dividends out of their retained earnings determined in accordance with the accounting standards and regulations in the PRC and after they have met the PRC requirements for appropriation to statutory reserve. In addition, most of the Company’s businesses and assets are denominated in RMB, which is not freely convertible into foreign currencies. All foreign exchange transactions take place either through the People’s Bank of China or other banks authorized to buy and sell foreign currencies at the exchange rates quoted by the People’s Bank of China. Approval of foreign currency payments by the People’s Bank of China or other regulatory institutions requires submitting a payment application form together with suppliers’ invoices, shipping documents and signed contracts. These currency exchange control procedures imposed by the PRC government authorities may restrict the ability of the Company’s PRC subsidiary to transfer their net assets to the Lonlon Biotech Ltd. (the “Parent Company”) through loans, advances or cash dividends.
Schedule I of Article 5-04 of Regulation S-X requires the condensed financial information of the parent company to be filed when the restricted net assets of consolidated subsidiaries exceed 25 percent of consolidated net assets as of the end of the most recently completed fiscal year. For purposes of this test, restricted net assets of consolidated subsidiaries shall mean that amount of the registrant’s proportionate share of net assets of its consolidated subsidiaries (after intercompany eliminations) which as of the end of the most recent fiscal year may not be transferred to the parent company in the form of loans, advances or cash dividends without the consent of a third party. The restricted net assets of the Company’s PRC subsidiary amounted to approximately $453,000 and $589,000 as of March 31, 2021 and December 31, 2020, respectively.
F-56
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 13 – RESTRICTED NET ASSETS (continued)
The Company’s PRC subsidiary’ net assets as of March 31, 2021 and December 31, 2020 exceeded 25% of the Company’s consolidated net assets. Accordingly, Parent Company’s condensed financial statements have been prepared in accordance with Rule 5-04 and Rule 12-04 of SEC Regulation S-X, and are as follows.
Condensed Financial Information of the Parent Company
The Parent Company’s condensed financial statements have been prepared using the same accounting principles and policies described in the notes to the consolidated financial statements, with the only exception being that the Parent Company accounts for its subsidiaries using the equity method. Refer to the consolidated financial statements and notes presented above for additional information and disclosures with respect to these financial statements.
| Parent Company’s Condensed Balance Sheets | ||||||||
| March 31, 2021 | December 31, 2020 | |||||||
| ASSETS | ||||||||
| NON-CURRENT ASSETS: | ||||||||
| Investment in subsidiaries | $ | 453,328 | $ | 589,185 | ||||
| Total Non-current Assets | 453,328 | 589,185 | ||||||
| Total Assets | $ | 453,328 | $ | 589,185 | ||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
| Total Liabilities | $ | - | $ | - | ||||
| Commitments and Contingencies | ||||||||
| SHAREHOLDERS’ EQUITY: | ||||||||
Ordinary shares, $1.00 par value; 50,000 shares authorized; 10,001 shares issued and outstanding at March 31, 2021 and December 2020 * | 10,001 | 10,001 | ||||||
| Additional paid-in capital | 8,946,197 | 8,946,197 | ||||||
| Accumulative deficit | (8,515,294 | ) | (8,380,014 | ) | ||||
| Accumulated other comprehensive income | 12,424 | 13,001 | ||||||
| Total Shareholders’ Equity | 453,328 | 589,185 | ||||||
| Total Liabilities and Shareholders’ Equity | $ | 453,328 | $ | 589,185 | ||||
| * | The shares amounts are presented on a retroactive basis. |
| Parent Company’s Condensed Statements of Operations and Comprehensive Loss | ||||||||
| For the Three Months Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| Revenue | $ | - | $ | - | ||||
| Operating expense | - | - | ||||||
| Income attributable to Parent Company only | - | - | ||||||
| Share of loss from investment in subsidiaries | (135,280 | ) | (208,672 | ) | ||||
| Net loss | (135,280 | ) | (208,672 | ) | ||||
| Other comprehensive loss | ||||||||
| Unrealized foreign currency translation loss | (577 | ) | (47,085 | ) | ||||
| Comprehensive loss | $ | (135,857 | ) | $ | (255,757 | ) | ||
F-57
LONLON BIOTECH LTD. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 13 – RESTRICTED NET ASSETS (continued)
Condensed Financial Information of the Parent Company (continued)
| Parent Company’s Condensed Statements of Cash Flows | ||||||||
| For the Three Months Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | (135,280 | ) | $ | (208,672 | ) | ||
| Adjustments to reconcile net loss to net cash provided by operating activities: | ||||||||
| Share of loss from investment in subsidiaries | 135,280 | 208,672 | ||||||
| NET CASH PROVIDED BY OPERATING ACTIVITIES | - | - | ||||||
| NET INCREASE IN CASH | - | - | ||||||
| CASH - beginning of period | - | - | ||||||
| CASH - end of period | $ | - | $ | - | ||||
Basis of Preparation
The condensed financial information of the Parent Company has been prepared using the same accounting policies as set out in the consolidated financial statements except that the Company used the equity method to account for investment in its subsidiaries.
Certain information and footnote disclosures normally included in financial statements prepared in conformity with accounting principles generally accepted in the United States of America have been condensed or omitted. The Parent Company only financial information has been derived from the Company’s consolidated financial statements and should be read in conjunction with the Company’s consolidated financial statements.
Investment in Subsidiaries
The Company and its subsidiaries were included in the consolidated financial statements where the inter-company balances and transactions were eliminated upon consolidation. For purpose of the Parent Company’s stand-alone financial statements, its investments in subsidiaries were reported using the equity method of accounting. Such investment is presented as “Investment in subsidiaries” on the condensed balance sheets and the subsidiaries’ loss is presented as “Share of loss from investment in subsidiaries” in the condensed statements of operations.
NOTE 14 – SUBSEQUENT EVENTS
In May 2021, the Company repaid a short-term loan from China Construction Bank in the principal amount of $457,827.
In May 2021, the Company borrowed a short-term loan from a related party company in the principal amount of $763,044. The new loan bears interest rate at 4.35% per annum and is due on October 31, 2021.
On April 26, 2021, Senlang BJ entered into a series of contractual arrangements, or VIE agreements with SenlangBio and 13 equity holders of SenlangBio, through which the Company obtained control and became the primary beneficiary of SenlangBio. As a result, SenlangBio became the Company’s VIE.
On June 13, 2021, the Company entered into a Share Purchase Agreement (the “Purchase Agreement”), by and among the Company, Avalon GloboCare Corp., a Delaware corporation (“Avalon”), the holders of the share capital of Senlang (the “Senlang Shareholders”), the ultimate beneficial owners of the Senlang Shareholders (the “Senlang Beneficial Shareholders” and, together with the Senlang Shareholders, the “Senlang Owners”) and a representative of the Senlang Owners (the “Senlang Representative”). Pursuant to the Purchase Agreement, Avalon will acquire all of the issued and outstanding share capital of the Company in consideration of 81 million shares of the common stock, par value $0.0001 per share, of Avalon (the “Acquisition”).
In connection with the Acquisition, on June 13, 2021, an institutional investor (the “Investor”) entered into an agreement with the related to the purchase of registered capital of the SenlangBio (the “SenlangBio Capital Increase Agreement”) pursuant to which the Investor will acquire an aggregate of up to 13.5% of the equity ownership of the SenlangBio for an aggregate purchase price of RMB 200,000,000 (approximately $30 million) (the “Equity Financing”), which funds will be invested in the SenlangBio in three installments of RMB 67,000,000 (approximately $10 million), RMB 67,000,000 (approximately $10 million) and RMB 66,000,000 (approximately $10 million), the first to be upon the closing of the Acquisition, the second to be within three months after the closing and the third to be within six months after the closing. In addition, pursuant to a Securities Exchange Agreement (the “Exchange Agreement”), by and among the Company, SenlangBio, Avalon and the Investor, dated June 13, 2021, the Investor has the right, exercisable between the six-month and five year-anniversaries of the respective initial closing and installment closings, to elect to exchange, from time to time, all or part of its then-owned equity ownership of SenlangBio for shares (the “Exchange Shares”) of Avalon Common Stock at a fixed exchange price of US$1.21 per share of Avalon Common Stock, which was the market price of the Avalon Common Stock as of the date of the Exchange Agreement under Nasdaq rules. In addition, the Exchange Agreement provides that the Investor may only exchange up to 10% of its total investment amount in any 30-day period.
These unaudited condensed consolidated financial statements were approved by management and available for issuance on June 24, 2021. The Company evaluated subsequent events through the date these unaudited condensed consolidated financial statements were issued.
F-58
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
CONDENSED CONSOLIDATED BALANCE SHEETS
| March 31, | December 31, | |||||||
| 2021 | 2020 | |||||||
| (Unaudited) | ||||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash | $ | 1,692,540 | $ | 726,577 | ||||
| Rent receivable | 23,471 | 35,395 | ||||||
| Deferred financing costs | 152,438 | 222,141 | ||||||
| Prepaid expenses and other current assets | 295,731 | 302,224 | ||||||
| Total Current Assets | 2,164,180 | 1,286,337 | ||||||
| NON-CURRENT ASSETS: | ||||||||
| Rent receivable - noncurrent portion | 109,174 | 111,840 | ||||||
| Security deposit | 19,662 | - | ||||||
| Deferred leasing costs | 133,359 | 144,197 | ||||||
| Operating lease right-of-use assets, net | 241,729 | 137,333 | ||||||
| Property and equipment, net | 439,962 | 479,115 | ||||||
| Investment in real estate, net | 7,644,950 | 7,685,686 | ||||||
| Equity method investment | 532,199 | 521,758 | ||||||
| Total Non-current Assets | 9,121,035 | 9,079,929 | ||||||
| Total Assets | $ | 11,285,215 | $ | 10,366,266 | ||||
| LIABILITIES AND EQUITY | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Accrued professional fees | $ | 1,445,225 | $ | 1,212,822 | ||||
| Accrued research and development fees | 432,500 | 513,533 | ||||||
| Accrued payroll liability and directors' compensation | 165,846 | 154,292 | ||||||
| Accrued liabilities and other payables | 344,453 | 367,411 | ||||||
| Accrued liabilities and other payables - related parties | 313,105 | 267,956 | ||||||
| Operating lease obligation | 132,657 | 76,379 | ||||||
| Note payable - related party | 390,000 | - | ||||||
| Total Current Liabilities | 3,223,786 | 2,592,393 | ||||||
| NON-CURRENT LIABILITIES: | ||||||||
| Operating lease obligation - noncurrent portion | 109,337 | 66,954 | ||||||
| Note payable - related party | - | 390,000 | ||||||
| Loan payable - related party | 3,305,249 | 3,200,000 | ||||||
| Total Non-current Liabilities | 3,414,586 | 3,656,954 | ||||||
| Total Liabilities | 6,638,372 | 6,249,347 | ||||||
| Commitments and Contingencies | ||||||||
| EQUITY: | ||||||||
| Preferred stock, $0.0001 par value; 10,000,000 shares authorized; | ||||||||
| no shares issued and outstanding at March 31, 2021 and December 31, 2020 | - | - | ||||||
| Common stock, $0.0001 par value; 490,000,000 shares authorized; | ||||||||
| 84,943,564 shares issued and 84,423,564 shares outstanding at March 31, 2021; | ||||||||
| 82,795,297 shares issued and 82,275,297 shares outstanding at December 31, 2020 | 8,494 | 8,279 | ||||||
| Additional paid-in capital | 49,755,996 | 46,856,447 | ||||||
| Less: common stock held in treasury, at cost; | ||||||||
| 520,000 shares at March 31, 2021 and December 31, 2020 | (522,500 | ) | (522,500 | ) | ||||
| Accumulated deficit | (44,408,493 | ) | (42,041,375 | ) | ||||
| Statutory reserve | 6,578 | 6,578 | ||||||
| Accumulated other comprehensive loss - foreign currency translation adjustment | (193,232 | ) | (190,510 | ) | ||||
| Total Avalon GloboCare Corp. stockholders' equity | 4,646,843 | 4,116,919 | ||||||
| Non-controlling interest | - | - | ||||||
| Total Equity | 4,646,843 | 4,116,919 | ||||||
| Total Liabilities and Equity | $ | 11,285,215 | $ | 10,366,266 | ||||
See accompanying notes to the condensed consolidated financial statements.
F-59
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(Unaudited)
| For
the Three Months Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| REVENUES | ||||||||
| Real property rental | $ | 289,774 | $ | 296,956 | ||||
| COSTS AND EXPENSES | ||||||||
| Real property operating expenses | 216,894 | 254,501 | ||||||
| GROSS PROFIT | ||||||||
| Real property operating income | 72,880 | 42,455 | ||||||
| OTHER OPERATING EXPENSES: | ||||||||
| Professional fees | 1,381,178 | 1,553,698 | ||||||
| Compensation and related benefits | 562,006 | 1,128,468 | ||||||
| Research and development expenses | 213,188 | 275,402 | ||||||
| Other general and administrative | 220,096 | 307,079 | ||||||
| Total Other Operating Expenses | 2,376,468 | 3,264,647 | ||||||
| LOSS FROM OPERATIONS | (2,303,588 | ) | (3,222,192 | ) | ||||
| OTHER INCOME (EXPENSE) | ||||||||
| Interest expense - related party | (45,149 | ) | (42,169 | ) | ||||
| Loss from equity method investment | (18,514 | ) | (9,084 | ) | ||||
| Other income | 133 | 2,664 | ||||||
| Total Other Expense, net | (63,530 | ) | (48,589 | ) | ||||
| LOSS BEFORE INCOME TAXES | (2,367,118 | ) | (3,270,781 | ) | ||||
| INCOME TAXES | - | - | ||||||
| NET LOSS | $ | (2,367,118 | ) | $ | (3,270,781 | ) | ||
| LESS: NET LOSS ATTRIBUTABLE TO NON-CONTROLLING INTEREST | - | - | ||||||
| NET LOSS ATTRIBUTABLE TO AVALON GLOBOCARE CORP. COMMON SHAREHOLDERS | $ | (2,367,118 | ) | $ | (3,270,781 | ) | ||
| COMPREHENSIVE LOSS: | ||||||||
| NET LOSS | $ | (2,367,118 | ) | $ | (3,270,781 | ) | ||
| OTHER COMPREHENSIVE LOSS | ||||||||
| Unrealized foreign currency translation loss | (2,722 | ) | (22,066 | ) | ||||
| COMPREHENSIVE LOSS | (2,369,840 | ) | (3,292,847 | ) | ||||
| LESS: COMPREHENSIVE LOSS ATTRIBUTABLE TO NON-CONTROLLING INTEREST | - | - | ||||||
| COMPREHENSIVE LOSS ATTRIBUTABLE TO AVALON GLOBOCARE CORP. COMMON SHAREHOLDERS | $ | (2,369,840 | ) | $ | (3,292,847 | ) | ||
| NET LOSS PER COMMON SHARE ATTRIBUTABLE TO AVALON GLOBOCARE CORP. COMMON SHAREHOLDERS: | ||||||||
| Basic and diluted | $ | (0.03 | ) | $ | (0.04 | ) | ||
| WEIGHTED AVERAGE COMMON SHARES OUTSTANDING: | ||||||||
| Basic and diluted | 83,413,154 | 76,712,054 | ||||||
See accompanying notes to the condensed consolidated financial statements.
F-60
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
For the Three Months Ended March 31, 2021
(Unaudited)
| Avalon GloboCare Corp. Stockholders' Equity | ||||||||||||||||||||||||||||||||||||||||||||||||
| Preferred Stock | Common Stock | Treasury Stock | Accumulated | |||||||||||||||||||||||||||||||||||||||||||||
| Number | Number | Additional | Number | Other | Non- | |||||||||||||||||||||||||||||||||||||||||||
| of | of | Paid-in | of | Accumulated | Statutory | Comprehensive | controlling | Total | ||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Shares | Amount | Deficit | Reserve | Loss | Interest | Equity | |||||||||||||||||||||||||||||||||||||
| Balance, January 1, 2021 | - | $ | - | 82,795,297 | $ | 8,279 | $ | 46,856,447 | (520,000 | ) | $ | (522,500 | ) | $ | (42,041,375 | ) | $ | 6,578 | $ | (190,510 | ) | $ | - | $ | 4,116,919 | |||||||||||||||||||||||
| Sale of common stock, net | - | - | 1,848,267 | 185 | 2,337,074 | - | - | - | - | - | - | 2,337,259 | ||||||||||||||||||||||||||||||||||||
| Issuance
of common stock for services (Note 8) | - | - | 300,000 | 30 | 359,970 | - | - | - | - | - | - | 360,000 | ||||||||||||||||||||||||||||||||||||
| Stock-based compensation | - | - | - | - | 202,505 | - | - | - | - | - | - | 202,505 | ||||||||||||||||||||||||||||||||||||
| Foreign
currency translation adjustment | - | - | - | - | - | - | - | - | - | (2,722 | ) | - | (2,722 | ) | ||||||||||||||||||||||||||||||||||
| Net
loss for the three months ended March 31, 2021 | - | - | - | - | - | - | - | (2,367,118 | ) | - | - | - | (2,367,118 | ) | ||||||||||||||||||||||||||||||||||
| Balance, March 31, 2021 | - | $ | - | 84,943,564 | $ | 8,494 | $ | 49,755,996 | (520,000 | ) | $ | (522,500 | ) | $ | (44,408,493 | ) | $ | 6,578 | $ | (193,232 | ) | $ | - | $ | 4,646,843 | |||||||||||||||||||||||
See accompanying notes to the condensed consolidated financial statements.
F-61
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
For the Three Months Ended March 31, 2020
(Unaudited)
| Avalon GloboCare Corp. Stockholders' Equity | ||||||||||||||||||||||||||||||||||||||||||||||||
| Preferred Stock | Common Stock | Treasury Stock | Accumulated | |||||||||||||||||||||||||||||||||||||||||||||
| Number | Number | Additional | Number | Other | Non- | |||||||||||||||||||||||||||||||||||||||||||
| of | of | Paid-in | of | Accumulated | Statutory | Comprehensive | controlling | Total | ||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Shares | Amount | Deficit | Reserve | Loss | Interest | Equity | |||||||||||||||||||||||||||||||||||||
| Balance, January 1, 2020 | - | $ | - | 76,730,802 | $ | 7,673 | $ | 34,593,006 | (520,000 | ) | $ | (522,500 | ) | $ | (29,361,937 | ) | $ | 6,578 | $ | (257,747 | ) | $ | - | $ | 4,465,073 | |||||||||||||||||||||||
| Sale of common stock, net | - | - | 980,358 | 98 | 1,539,153 | - | - | - | - | - | - | 1,539,251 | ||||||||||||||||||||||||||||||||||||
| Issuance of common stock for services | - | - | 222,577 | 22 | 213,278 | - | - | - | - | - | - | 213,300 | ||||||||||||||||||||||||||||||||||||
| Stock-based compensation | - | - | - | - | 785,350 | - | - | - | - | - | - | 785,350 | ||||||||||||||||||||||||||||||||||||
| Foreign currency translation adjustment | - | - | - | - | - | - | - | - | - | (22,066 | ) | - | (22,066 | ) | ||||||||||||||||||||||||||||||||||
| Net loss for the three months ended March 31, 2020 | - | - | - | - | - | - | - | (3,270,781 | ) | - | - | - | (3,270,781 | ) | ||||||||||||||||||||||||||||||||||
| Balance, March 31, 2020 | - | $ | - | 77,933,737 | $ | 7,793 | $ | 37,130,787 | (520,000 | ) | $ | (522,500 | ) | $ | (32,632,718 | ) | $ | 6,578 | $ | (279,813 | ) | $ | - | $ | 3,710,127 | |||||||||||||||||||||||
See accompanying notes to the condensed consolidated financial statements.
F-62
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(Unaudited)
| For the Three Months Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | (2,367,118 | ) | $ | (3,270,781 | ) | ||
| Adjustments to reconcile net loss to | ||||||||
| net cash used in operating activities: | ||||||||
| Bad debt provision | - | 4,698 | ||||||
| Depreciation | 78,875 | 76,535 | ||||||
| Amortization of straight-line rent receivable | 943 | 16,988 | ||||||
| Amortization of right-of-use asset | 27,531 | - | ||||||
| Stock-based compensation and service expense | 573,982 | 1,189,101 | ||||||
| Loss on equity method investment | 18,514 | 9,084 | ||||||
| Loss on fixed assets disposal | - | 2,648 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivable - related party | - | 85,932 | ||||||
| Rent receivable | 13,647 | (23,733 | ) | |||||
| Security deposit | 6,003 | - | ||||||
| Deferred leasing costs | (2,364 | ) | - | |||||
| Prepaid expenses and other current assets | (40,803 | ) | (96,669 | ) | ||||
| Accrued liabilities and other payables | 163,442 | (38,817 | ) | |||||
| Accrued liabilities and other payables - related parties | 45,149 | 28,218 | ||||||
| Operating lease obligation | (33,326 | ) | 18,000 | |||||
| NET CASH USED IN OPERATING ACTIVITIES | (1,515,525 | ) | (1,998,796 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Additional investment in equity method investment | (30,844 | ) | - | |||||
| NET CASH USED IN INVESTING ACTIVITIES | (30,844 | ) | - | |||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Proceeds received from loan payable - related party | 105,249 | 300,000 | ||||||
| Proceeds received from equity offering | 2,481,405 | 1,623,584 | ||||||
| Disbursements for equity offering costs | (74,442 | ) | (48,707 | ) | ||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES | 2,512,212 | 1,874,877 | ||||||
| EFFECT OF EXCHANGE RATE ON CASH | 120 | (5,701 | ) | |||||
| NET INCREASE (DECREASE) IN CASH | 965,963 | (129,620 | ) | |||||
| CASH - beginning of period | 726,577 | 764,891 | ||||||
| CASH - end of period | $ | 1,692,540 | $ | 635,271 | ||||
| NON-CASH INVESTING AND FINANCING ACTIVITIES: | ||||||||
| Common stock issued for future services | $ | - | $ | 57,207 | ||||
| Common stock issued for accrued liabilities | $ | 261,032 | $ | - | ||||
See accompanying notes to the condensed consolidated financial statements.
F-63
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 1 – ORGANIZATION AND NATURE OF OPERATIONS
Avalon GloboCare Corp. (the “Company” or “AVCO”) is a Delaware corporation. The Company was incorporated under the laws of the State of Delaware on July 28, 2014. On October 19, 2016, the Company entered into and closed a Share Exchange Agreement with the shareholders of Avalon Healthcare System, Inc., a Delaware corporation (“AHS”), each of which were accredited investors (“AHS Shareholders”) pursuant to which we acquired 100% of the outstanding securities of AHS in exchange for 50,000,000 shares of the Company’s common stock (the “AHS Acquisition”). AHS was incorporated on May 18, 2015 under the laws of the State of Delaware.
For accounting purposes, AHS was the surviving entity. The transaction was accounted for as a recapitalization of AHS pursuant to which AHS was treated as the accounting acquirer, surviving and continuing entity although the Company is the legal acquirer. The Company did not recognize goodwill or any intangible assets in connection with this transaction. Accordingly, the Company’s historical financial statements are those of AHS and its wholly-owned subsidiary, Avalon (Shanghai) Healthcare Technology Co., Ltd. (“Avalon Shanghai”) immediately following the consummation of this reverse merger transaction. AHS owns 100% of the capital stock of Avalon Shanghai, which is a wholly foreign-owned enterprise organized under the laws of the People’s Republic of China (“PRC”). Avalon Shanghai was incorporated on April 29, 2016 and is engaged in medical related consulting services for customers.
The Company is a clinical-stage, vertically integrated, leading CellTech bio-developer dedicated to advancing and empowering innovative, transformative immune effector cell therapy, exosome technology, as well as COVID-19 related diagnostics and therapeutics. The Company also provides strategic advisory and outsourcing services to facilitate and enhance its clients' growth and development, as well as competitiveness in healthcare and CellTech industry markets. Through its subsidiary structure with unique integration of verticals from innovative R&D to automated bioproduction and accelerated clinical development, the Company is establishing a leading role in the fields of cellular immunotherapy (including CAR-T/NK), exosome technology (ACTEX™), and regenerative therapeutics.
On January 23, 2017, the Company incorporated Avalon (BVI) Ltd., a British Virgin Island company. There was no activity for the subsidiary since its incorporation through March 31, 2021. Avalon (BVI) Ltd. is dormant and is in process of being dissolved.
On February 7, 2017, the Company formed Avalon RT 9 Properties, LLC (“Avalon RT 9”), a New Jersey limited liability company. On May 5, 2017, Avalon RT 9 purchased a real property located in Township of Freehold, County of Monmouth, State of New Jersey, having a street address of 4400 Route 9 South, Freehold, NJ 07728. This property was purchased to serve as the Company’s world-wide headquarters for all corporate administration and operations. In addition, the property generates rental income. Avalon RT 9 owns this office building. Currently, Avalon RT 9’s business consists of the ownership and operation of the income-producing real estate property in New Jersey. As of March 31, 2021, the occupancy rate of the building is 89.4%.
On July 31, 2017, the Company formed Genexosome Technologies Inc. (“Genexosome”) in Nevada. Genexosome was engaged in developing proprietary diagnostic and therapeutic products using exosomes. Genexosome owns 100% of the capital stock of Beijing Jieteng (Genexosome) Biotech Co., Ltd., a corporation incorporated in the People’s Republic of China on August 7, 2015 (“Beijing Genexosome”), and the Company holds 60% of Genexosome and Dr. Yu Zhou holds 40% of Genexosome. The Company had not been able to realize the financial projections provided by Dr. Zhou at the time of the acquisition and has decided to impair the intangible asset associated with this acquisition to zero. Dr. Zhou was terminated as Co-CEO of Genexosome on August 14, 2019. Since the fourth quarter of 2019, the non-controlling interest keeps inactive.
On July 18, 2018, the Company formed a wholly owned subsidiary, Avactis Biosciences Inc., a Nevada corporation, which will focus on accelerating commercial activities related to cellular therapies, including regenerative medicine with stem/progenitor cells as well as cellular immunotherapy including CAR-T, CAR-NK, TCR-T and others. The subsidiary is designed to integrate and optimize our global scientific and clinical resources to further advance the use of cellular therapies to treat certain cancers.
On June 13, 2019, the Company formed a wholly owned subsidiary, International Exosome Association LLC, a Delaware company. There was no activity for the subsidiary since its incorporation through March 31, 2021.
F-64
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 1 – ORGANIZATION AND NATURE OF OPERATIONS (continued)
Details of the Company’s subsidiaries which are included in these condensed consolidated financial statements as of March 31, 2021 are as follows:
| Name of Subsidiary | Place and date of Incorporation | Percentage of Ownership | Principal Activities | |||
Avalon Healthcare System, Inc. (“AHS”) |
Delaware May 18, 2015 |
100% held by AVCO | Provides medical related consulting services and developing Avalon Cell and Avalon Rehab in United States of America (“USA”) | |||
Avalon (BVI) Ltd. (“Avalon BVI”) |
British Virgin Island January 23, 2017 |
100% held by AVCO | Dormant, is in process of being dissolved | |||
Avalon RT 9 Properties LLC (“Avalon RT 9”) |
New Jersey February 7, 2017 |
100% held by AVCO | Owns and operates an income-producing real property and holds and manages the corporate headquarters | |||
Avalon (Shanghai) Healthcare Technology Co., Ltd. (“Avalon Shanghai”) |
PRC April 29, 2016 |
100% held by AHS | Provides medical related consulting services and developing Avalon Cell and Avalon Rehab in China | |||
Genexosome Technologies Inc. (“Genexosome”) |
Nevada July 31, 2017 |
60% held by AVCO | Dormant | |||
Beijing Jieteng (Genexosome) Biotech Co., Ltd. (“Beijing Genexosome”) |
PRC August 7, 2015 |
100% held by Genexosome | Provides development services for hospitals and other customers and sells developed items to hospitals and other customers in China | |||
Avactis Biosciences Inc. (“Avactis”) |
Nevada July 18, 2018 |
100% held by AVCO | Integrate and optimize global scientific and clinical resources to further advance cellular therapies, including regenerative medicine with stem/progenitor cells as well as cellular immunotherapy including CAR-T, CAR-NK, TCR-T and others to treat certain cancers | |||
International Exosome Association LLC (“Exosome”) |
Delaware June 13, 2019 |
100% held by AVCO | Promotes standardization related to exosome industry |
NOTE 2 – BASIS OF PRESENTATION AND GOING CONCERN CONDITION
Basis of Presentation
These interim condensed consolidated financial statements of the Company and its subsidiaries are unaudited. In the opinion of management, all adjustments (consisting of normal recurring accruals) and disclosures necessary for a fair presentation of these interim condensed consolidated financial statements have been included. The results reported in the condensed consolidated financial statements for any interim periods are not necessarily indicative of the results that may be reported for the entire year. The accompanying condensed consolidated financial statements have been prepared in accordance with the rules and regulations of the Securities and Exchange Commission and do not include all information and footnotes necessary for a complete presentation of financial statements in conformity with accounting principles generally accepted in the United States (“U.S. GAAP”). The Company’s condensed consolidated financial statements include the accounts of the Company and its subsidiaries. All significant intercompany accounts and transactions have been eliminated in consolidation.
Certain information and footnote disclosures normally included in the annual consolidated financial statements prepared in accordance with U.S. GAAP have been condensed or omitted. These condensed consolidated financial statements should be read in conjunction with the Company’s audited consolidated financial statements and notes thereto included in the Company’s Annual Report on Form 10-K for the year ended December 31, 2020 filed with the Securities and Exchange Commission on March 30, 2021.
F-65
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 2 – BASIS OF PRESENTATION AND GOING CONCERN CONDITION (continued)
Going Concern
The Company is a clinical-stage, vertically integrated, leading CellTech bio-developer dedicated to advancing and empowering innovative, transformative immune effector cell therapy, exosome technology, as well as COVID-19 related diagnostics and therapeutics. The Company also provides strategic advisory and outsourcing services to facilitate and enhance its clients' growth and development, as well as competitiveness in healthcare and CellTech industry markets. Through its subsidiary structure with unique integration of verticals from innovative R&D to automated bioproduction and accelerated clinical development, the Company is establishing a leading role in the fields of cellular immunotherapy (including CAR-T/NK), exosome technology (ACTEX™), and regenerative therapeutics.
In addition, the Company owns commercial real estate that houses its headquarters in Freehold, New Jersey and provides outsourced, customized international healthcare services to the rapidly changing health care industry primarily focused in the People’s Republic of China. The Company did not generate any revenue from medical related consulting services segment during the three months ended March 31, 2021. These condensed consolidated financial statements have been prepared assuming that the Company will continue as a going concern, which contemplates, among other things, the realization of assets and the satisfaction of liabilities in the normal course of business.
As reflected in the accompanying condensed consolidated financial statements, the Company had working capital deficit of $1,059,606 as of March 31, 2021 and has incurred recurring net loss and generated negative cash flow from operating activities of $2,367,118 and $1,515,525 for the three months ended March 31, 2021, respectively. The Company has a limited operating history and its continued growth is dependent upon the continuation of providing medical consulting services to its only few clients who are related parties and generating rental revenue from its income-producing real estate property in New Jersey; hence generating revenues, and obtaining additional financing to fund future obligations and pay liabilities arising from normal business operations. In addition, the current cash balance cannot be projected to cover the operating expenses for the next twelve months from the release date of this report. These matters raise substantial doubt about the Company’s ability to continue as a going concern. The ability of the Company to continue as a going concern is dependent on the Company’s ability to raise additional capital, implement its business plan, and generate significant revenues. There are no assurances that the Company will be successful in its efforts to generate significant revenues, maintain sufficient cash balance or report profitable operations or to continue as a going concern. The Company plans on raising capital through the sale of equity to implement its business plan. However, there is no assurance these plans will be realized and that any additional financings will be available to the Company on satisfactory terms and conditions, if any.
The occurrence of an uncontrollable event such as the COVID-19 pandemic had negatively impact on the Company’s operations. Some tenants have delayed on rent payment. Our general development operations have continued during the COVID-19 pandemic and we have not had significant disruption. However, we are uncertain if the COVID-19 pandemic will impact future operations at our laboratory, or our ability to collaborate with other laboratories and universities. In addition, we are unsure if the COVID-19 pandemic will impact future clinical trials. Given the dynamic nature of these circumstances, the duration of business disruption and reduced traffic, the related financial effect cannot be reasonably estimated at this time but is expected to adversely impact the Company’s business for the rest of 2021.
The accompanying condensed consolidated financial statements do not include any adjustments related to the recoverability or classification of asset-carrying amounts or the amounts and classification of liabilities that may result should the Company be unable to continue as a going concern.
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Use of Estimates
The preparation of the condensed consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from these estimates. Significant estimates during the three months ended March 31, 2021 and 2020 include the useful life of property and equipment and investment in real estate, assumptions used in assessing impairment of long-term assets, valuation of deferred tax assets and the associated valuation allowances, and valuation of stock-based compensation.
F-66
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Fair Value of Financial Instruments and Fair Value Measurements
The Company adopted the guidance of Accounting Standards Codification (“ASC”) 820 for fair value measurements which clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
| ● | Level 1-Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date. |
| ● | Level 2-Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other than quoted prices that are observable, and inputs derived from or corroborated by observable market data. |
| ● | Level 3-Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information. |
The carrying amounts reported in the condensed consolidated balance sheets for cash, rent receivable, accrued liabilities and other payables, accrued liabilities and other payables – related parties, operating lease obligation, and note payable, approximate their fair market value as of March 31, 2021 and December 31, 2020 based on the short-term maturity of these instruments.
ASC 825-10 “Financial Instruments”, allows entities to voluntarily choose to measure certain financial assets and liabilities at fair value (fair value option). The fair value option may be elected on an instrument-by-instrument basis and is irrevocable, unless a new election date occurs. If the fair value option is elected for an instrument, unrealized gains and losses for that instrument should be reported in earnings at each subsequent reporting date. The Company did not elect to apply the fair value option to any outstanding instruments.
Cash and Cash Equivalents
At March 31, 2021 and December 31, 2020, the Company’s cash balances by geographic area were as follows:
| Country: | March 31, 2021 | December 31, 2020 | ||||||||||||||
| United States | $ | 1,527,257 | 90.2 | % | $ | 559,711 | 77.0 | % | ||||||||
| China | 165,283 | 9.8 | % | 166,866 | 23.0 | % | ||||||||||
| Total cash | $ | 1,692,540 | 100.0 | % | $ | 726,577 | 100.0 | % | ||||||||
For purposes of the condensed consolidated statements of cash flows, the Company considers all highly liquid instruments with a maturity of three months or less when purchased and money market accounts to be cash equivalents. The Company had no cash equivalents at March 31, 2021 and December 31, 2020.
Credit Risk and Uncertainties
A portion of the Company’s cash is maintained with state-owned banks within the PRC. Balances at state-owned banks within the PRC are covered by insurance up to RMB 500,000 (approximately $76,000) per bank. Any balance over RMB 500,000 per bank in PRC will not be covered. At March 31, 2021, cash balances held in the PRC are RMB 1,083,049 (approximately $165,000), of which, RMB 556,726 (approximately $85,000) was not covered by such limited insurance. The Company has not experienced any losses in such accounts and believes it is not exposed to any risks on its cash in bank accounts.
The Company maintains a portion of its cash in bank and financial institution deposits within U.S. that at times may exceed federally-insured limits of $250,000. The Company manages this credit risk by concentrating its cash balances in high quality financial institutions and by periodically evaluating the credit quality of the primary financial institutions holding such deposits. The Company has not experienced any losses in such bank accounts and believes it is not exposed to any risks on its cash in bank accounts. At March 31, 2021, the Company’s cash balances in United States bank accounts had approximately $986,000 in excess of the federally-insured limits.
F-67
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Credit Risk and Uncertainties (continued)
Currently, a portion of the Company’s operations are carried out in PRC. Accordingly, the Company’s business, financial condition and results of operations may be influenced by the political, economic and legal environment in the PRC, and by the general state of the PRC’s economy. The Company’s operations in PRC are subject to specific considerations and significant risks not typically associated with companies in North America. The Company’s results may be adversely affected by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
Financial instruments which potentially subject the Company to concentrations of credit risk consist principally of trade accounts receivable. A portion of the Company’s sales are credit sales which is to the customer whose ability to pay is dependent upon the industry economics prevailing in these areas; however, concentrations of credit risk with respect to trade accounts receivable is limited due to short-term payment terms. The Company also performs ongoing credit evaluations of its customers to help further reduce credit risk.
Investment in Unconsolidated Company – Epicon Biosciences Co., Ltd.
The Company uses the equity method of accounting for its investment in, and earning or loss of, company that it does not control but over which it does exert significant influence. The Company considers whether the fair value of its equity method investment has declined below its carrying value whenever adverse events or changes in circumstances indicate that recorded value may not be recoverable. If the Company considers any decline to be other than temporary (based on various factors, including historical financial results and the overall health of the investee), then a write-down would be recorded to estimated fair value. See Note 5 for discussion of equity method investment.
Revenue Recognition
The Company recognizes revenue under Accounting Standards Codification (“ASC”) Topic 606, Revenue from Contracts with Customers (“ASC 606”). The core principle of the revenue standard is that a company should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. The following five steps are applied to achieve that core principle:
| ● | Step 1: Identify the contract with the customer |
| ● | Step 2: Identify the performance obligations in the contract |
| ● | Step 3: Determine the transaction price |
| ● | Step 4: Allocate the transaction price to the performance obligations in the contract |
| ● | Step 5: Recognize revenue when the company satisfies a performance obligation |
In order to identify the performance obligations in a contract with a customer, a company must assess the promised goods or services in the contract and identify each promised goods or service that is distinct. A performance obligation meets ASC 606’s definition of a “distinct” goods or service (or bundle of goods or services) if both of the following criteria are met:
| ● | The customer can benefit from the goods or service either on its own or together with other resources that are readily available to the customer (i.e., the goods or service is capable of being distinct). |
| ● | The entity’s promise to transfer the goods or service to the customer is separately identifiable from other promises in the contract (i.e., the promise to transfer the goods or service is distinct within the context of the contract). |
If a goods or service is not distinct, the goods or service is combined with other promised goods or services until a bundle of goods or services is identified that is distinct.
F-68
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Revenue Recognition (continued)
The transaction price is the amount of consideration to which an entity expects to be entitled in exchange for transferring promised goods or services to a customer, excluding amounts collected on behalf of third parties (for example, some sales taxes). The consideration promised in a contract with a customer may include fixed amounts, variable amounts, or both. Variable consideration is included in the transaction price only to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved.
The transaction price is allocated to each performance obligation on a relative standalone selling price basis. The transaction price allocated to each performance obligation is recognized when that performance obligation is satisfied, at a point in time or over time as appropriate.
The Company’s revenues are derived from providing medial related consulting services for its’ related parties. Revenues related to its service offerings are recognized as the services are performed. Any payments received in advance of the performance of services are recorded as deferred revenue until such time as the services are performed.
The Company has determined that the ASC 606 does not apply to rental contracts, which are within the scope of other revenue recognition accounting standards.
Rental income from operating leases is recognized on a straight-line basis under the guidance of ASC 842. Lease payments under tenant leases are recognized on a straight-line basis over the term of the related leases. The cumulative difference between lease revenue recognized under the straight-line method and contractual lease payments are included in rent receivable on the condensed consolidated balance sheets.
The Company does not offer promotional payments, customer coupons, rebates or other cash redemption offers to its customers.
Per Share Data
ASC Topic 260 “Earnings per Share,” requires presentation of both basic and diluted earnings per share (“EPS”) with a reconciliation of the numerator and denominator of the basic EPS computation to the numerator and denominator of the diluted EPS computation. Basic EPS excludes dilution. Diluted EPS reflects the potential dilution that could occur if securities or other contracts to issue common stock were exercised or converted into common stock or resulted in the issuance of common stock that then shared in the earnings of the entity.
Basic net loss per share is computed by dividing net loss available to common stockholders by the weighted average number of shares of common stock outstanding during the period. Diluted net loss per share is computed by dividing net loss by the weighted average number of shares of common stock, common stock equivalents and potentially dilutive securities outstanding during each period. For the three months ended March 31, 2021 and 2020, potentially dilutive common shares consist of the common shares issuable upon the exercise of common stock options (using the treasury stock method). Common stock equivalents are not included in the calculation of diluted net loss per share if their effect would be anti-dilutive. In a period in which the Company has a net loss, all potentially dilutive securities are excluded from the computation of diluted shares outstanding as they would have had an anti-dilutive impact.
The following table summarizes the securities that were excluded from the diluted per share calculation because the effect of including these potential shares was antidilutive:
| Three Months Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| Stock options | 7,580,000 | 6,800,000 | ||||||
| Potentially dilutive securities | 7,580,000 | 6,800,000 | ||||||
F-69
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Segment Reporting
The Company uses “the management approach” in determining reportable operating segments. The management approach considers the internal organization and reporting used by the Company’s chief operating decision maker for making operating decisions and assessing performance as the source for determining the Company’s reportable segments. The Company’s chief operating decision maker is the Chief Executive Officer (“CEO”) and president of the Company, who reviews operating results to make decisions about allocating resources and assessing performance for the entire Company.
The Company previously had three reportable business segments: real property operating segment, medical related consulting services segment, and development services and sales of developed products segment. Due to the winding down of the development services and sales of developed products segment in 2020, the Company no longer has any material revenues or expenses in this segment. As a result, commencing from the first quarter of 2021, the Company’s chief operating decision maker no longer reviews development services and sales of developed products operating results and the Company no longer reports in three segments.
During the three months ended March 31, 2021, the Company operates through two business segments: real property operating segment and medical related consulting services segment. These reportable segments offer different types of services and products, have different types of revenue, and are managed separately as each requires different operating strategies and management expertise.
Reclassification
Certain prior period amounts have been reclassified to conform to the current period presentation. These reclassifications have no effect on the previously reported financial position, results of operations and cash flows.
Recent Accounting Standards
In June 2016, the FASB issued ASU 2016-13, Financial Instruments - Credit Losses (“Topic 326”). The ASU introduces a new accounting model, the Current Expected Credit Losses model (“CECL”), which requires earlier recognition of credit losses and additional disclosures related to credit risk. The CECL model utilizes a lifetime expected credit loss measurement objective for the recognition of credit losses at the time the financial asset is originated or acquired. ASU 2016-13 is effective for annual period beginning after December 15, 2022, including interim reporting periods within those annual reporting periods. The Company expects that the adoption will not have a material impact on the Company’s consolidated financial statements.
In December 2019, the FASB issued ASU 2019-12, Simplifying the Accounting for Income Taxes, as part of its Simplification Initiative to reduce the cost and complexity in accounting for income taxes. This standard removes certain exceptions related to the approach for intra period tax allocation, the methodology for calculating income taxes in an interim period and the recognition of deferred tax liabilities for outside basis differences. It also amends other aspects of the guidance to help simplify and promote consistent application of GAAP. The guidance is effective for interim and annual periods beginning after December 15, 2020, with early adoption permitted. The adoption of ASU 2019 – 12 did not have a material impact on the Company’s consolidated financial statements.
Other accounting standards that have been issued or proposed by FASB that do not require adoption until a future date are not expected to have a material impact on the consolidated financial statements upon adoption. The Company does not discuss recent pronouncements that are not anticipated to have an impact on or are unrelated to its consolidated financial condition, results of operations, cash flows or disclosures.
F-70
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 4 – PREPAID EXPENSES AND OTHER CURRENT ASSETS
At March 31, 2021 and December 31, 2020, prepaid expenses and other current assets consisted of the following:
| March 31, 2021 | December 31, 2020 | |||||||
| Prepaid NASDAQ listing fee | $ | 63,417 | $ | - | ||||
| Prepaid directors and officers liability insurance premium | 62,038 | 64,929 | ||||||
| Prepaid professional fees | 53,364 | 78,639 | ||||||
| Recoverable VAT | 41,934 | 40,446 | ||||||
| Deferred leasing costs | 31,422 | 18,220 | ||||||
| Prepaid research and development fees | 30,305 | 60,610 | ||||||
| Other | 13,251 | 39,380 | ||||||
| Total | $ | 295,731 | $ | 302,224 | ||||
NOTE 5 – EQUITY METHOD INVESTMENT
As of March 31, 2021 and December 31, 2020, the equity method investment amounted to $532,199 and $521,758, respectively. The investment represents the Company’s subsidiary, Avalon Shanghai’s interest in Epicon Biotech Co., Ltd. (“Epicon”). Epicon was incorporated on August 14, 2018 in PRC. Avalon Shanghai and the other unrelated company, Jiangsu Unicorn Biological Technology Co., Ltd. (“Unicorn”), accounted for 40% and 60% of the total ownership, respectively. Epicon is focused on cell preparation, third party testing, biological sample repository for commercial and scientific research purposes and the clinical transformation of scientific achievements.
The Company treats the equity investment in the consolidated financial statements under the equity method. Under the equity method, the investment is initially recorded at cost, adjusted for any excess of the Company’s share of the incorporated-date fair values of the investee’s identifiable net assets over the cost of the investment (if any). Thereafter, the investment is adjusted for the post incorporation change in the Company’s share of the investee’s net assets and any impairment loss relating to the investment.
For the three months ended March 31, 2021 and 2020, the Company’s share of Epicon’s net loss was $18,514 and $9,084, respectively, which was included in loss from equity method investment in the accompanying condensed consolidated statements of operations and comprehensive loss.
In the three months ended March 31, 2021, activity recorded for the Company’s equity method investment in Epicon is summarized in the following table:
| Equity investment carrying amount at January 1, 2021 | $ | 521,758 | ||
| Payment made for equity method investment | 30,844 | |||
| Epicon's net loss attributable to the Company | (18,514 | ) | ||
| Foreign currency fluctuation | (1,889 | ) | ||
| Equity investment carrying amount at March 31, 2021 | $ | 532,199 |
F-71
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 5 – EQUITY METHOD INVESTMENT (continued)
The tables below present the summarized financial information, as provided to the Company by the investee, for the unconsolidated company:
| March 31, 2021 | December 31, 2020 | |||||||
| Current assets | $ | 13,071 | $ | 13,023 | ||||
| Noncurrent assets | 255,884 | 264,390 | ||||||
| Current liabilities | 14,351 | 6,615 | ||||||
| Noncurrent liabilities | - | - | ||||||
| Equity | 254,604 | 270,798 | ||||||
| For the Three Months Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| Net revenue | $ | - | $ | - | ||||
| Gross profit | - | - | ||||||
| Loss from operation | 46,286 | 22,711 | ||||||
| Net loss | 46,286 | 22,711 | ||||||
NOTE 6 – ACCRUED LIABILITIES AND OTHER PAYABLES
At March 31, 2021 and December 31, 2020, accrued liabilities and other payables consisted of the following:
| March 31, 2021 | December 31, 2020 | |||||||
| Accrued professional fees | $ | 1,445,225 | $ | 1,212,822 | ||||
| Accrued research and development fees | 432,500 | 513,533 | ||||||
| Accrued payroll liability and directors’ compensation | 165,846 | 154,292 | ||||||
| Accrued tenants’ improvement reimbursement | 81,900 | 81,900 | ||||||
| Tenants’ security deposit | 69,533 | 69,634 | ||||||
| Accounts payable | 56,374 | 87,190 | ||||||
| Deferred rental income | 26,286 | 23,510 | ||||||
| Other | 110,360 | 105,177 | ||||||
| Total | $ | 2,388,024 | $ | 2,248,058 | ||||
NOTE 7 – RELATED PARTY TRANSACTIONS
Accrued Liabilities and Other Payables – Related Parties
The Company acquired Beijing Genexosome for a cash payment of $450,000. As of March 31, 2021 and December 31, 2020, the unpaid acquisition consideration of $100,000, was payable to Dr. Yu Zhou, former director and former co-chief executive officer and 40% owner of Genexosome, and has been included in accrued liabilities and other payables – related parties on the accompanying condensed consolidated balance sheets.
As of March 31, 2021 and December 31, 2020, the accrued and unpaid interest related to borrowings from Wenzhao Lu, the Company’s largest shareholder and chairman of the Board of Directors, amounted to $213,105 and $167,956, respectively, and have been included in accrued liabilities and other payables – related parties on the accompanying condensed consolidated balance sheets.
Borrowings from Related Party
Promissory Note
On March 18, 2019, the Company issued Wenzhao Lu, the Company’s largest shareholder and Chairman of the Board of Directors, a Promissory Note in the principal amount of $1,000,000 (“Promissory Note”) in consideration of cash in the amount of $1,000,000. The Promissory Note accrues interest at the rate of 5% per annum and matures March 19, 2022. The Company repaid principal of $410,000 and $200,000 in the third quarter of 2019 and second quarter of 2020, respectively. As of both March 31, 2021 and December 31, 2020, the outstanding principal balance was $390,000.
F-72
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 7 – RELATED PARTY TRANSACTIONS (continued)
Borrowings from Related Party (continued)
Line of Credit
On August 29, 2019, the Company entered into a Line of Credit Agreement (the “Line of Credit Agreement”) providing the Company with a $20 million line of credit (the “Line of Credit”) from Wenzhao Lu (the “Lender”), the largest shareholder and Chairman of the Board of Directors of the Company. The Line of Credit allows the Company to request loans thereunder and to use the proceeds of such loans for working capital and operating expense purposes until the facility matures on December 31, 2024. The loans are unsecured and are not convertible into equity of the Company. Loans drawn under the Line of Credit bears interest at an annual rate of 5% and each individual loan will be payable three years from the date of issuance. The Company has a right to draw down on the line of credit and not at the discretion of the related party Lender. The Company may, at its option, prepay any borrowings under the Line of Credit, in whole or in part at any time prior to maturity, without premium or penalty. The Line of Credit Agreement includes customary events of default. If any such event of default occurs, the Lender may declare all outstanding loans under the Line of Credit to be due and payable immediately. As of March 31, 2021 and December 31, 2020, $3,305,249 and $3,200,000 was outstanding under the Line of Credit, respectively.
For the three months ended March 31, 2021 and 2020, the interest expense related to above borrowings amounted to $45,149 and $42,169, respectively, and has been included in interest expense – related party on the accompanying condensed consolidated statements of operations and comprehensive loss.
As of March 31, 2021 and December 31, 2020, the related accrued and unpaid interest for above borrowings was $213,105 and $167,956, respectively, and has been included in accrued liabilities and other payables – related parties on the accompanying condensed consolidated balance sheets.
Office Space from Related Party
Beijing Genexosome uses office space of a related party, free of rent, which is considered immaterial.
NOTE 8 – EQUITY
2020 Incentive Stock Plan
The Company held its annual meeting on August 4, 2020. During its annual meeting, the Company approved 2020 Incentive Stock Plan and reserved 5,000,000 shares of common stock for issuance thereunder.
Common Shares Sold for Cash
On December 13, 2019, the Company entered into an Open Market Sale AgreementSM (the “Sales Agreement”) with Jefferies LLC, as sales agent (“Jefferies”), pursuant to which the Company may offer and sell, from time to time, through Jefferies, shares of its common stock. During the three months ended March 31, 2021, Jefferies sold an aggregate of 1,848,267 shares of common stock at an average price of $1.34 per share to investors. The Company recorded net proceeds of $2,337,259, net of commission and other offering costs of $144,146.
Common Shares Issued for Services
During the three months ended March 31, 2021, the Company issued a total of 300,000 shares of its common stock for services rendered. These shares were valued at $360,000, the fair market values on the grant dates using the reported closing share prices on the dates of grant and the Company recorded stock-based compensation expense of $98,968 for the three months ended March 31, 2021 and reduced accrued liabilities of $261,032.
F-73
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 8 – EQUITY (continued)
Options
The following table summarizes the shares of the Company’s common stock issuable upon exercise of options outstanding at March 31, 2021:
| Options Outstanding | Options Exercisable | |||||||||||||||||||||
| Range of Exercise Price | Number Outstanding at March 31, 2021 | Weighted Average Remaining Contractual Life (Years) | Weighted Average Exercise Price | Number Exercisable at March 31, 2021 | Weighted Average Exercise Price | |||||||||||||||||
| $ | 0.50 | 2,000,000 | 5.86 | $ | 0.50 | 2,000,000 | $ | 0.50 | ||||||||||||||
| 1.00 – 1.93 | 2,810,000 | 5.37 | 1.40 | 2,366,666 | 1.45 | |||||||||||||||||
| 2.00 – 2.80 | 2,740,000 | 2.52 | 2.17 | 2,740,000 | 2.17 | |||||||||||||||||
| 4.76 | 30,000 | 3.01 | 4.76 | 30,000 | 4.76 | |||||||||||||||||
| $ | 0.50 – 4.76 | 7,580,000 | 4.46 | $ | 1.45 | 7,136,666 | $ | 1.47 | ||||||||||||||
Stock option activities for the three months ended March 31, 2021 were as follows:
| Number of Options | Weighted Average Exercise Price | |||||||
| Outstanding at January 1, 2021 | 7,140,000 | $ | 1.48 | |||||
| Granted | 440,000 | 1.12 | ||||||
| Terminated / Exercised / Expired | - | - | ||||||
| Outstanding at March 31, 2021 | 7,580,000 | $ | 1.45 | |||||
| Options exercisable at March 31, 2021 | 7,136,666 | $ | 1.47 | |||||
| Options expected to vest | 443,334 | $ | 1.14 | |||||
The aggregate intrinsic value of both stock options outstanding and stock options exercisable at March 31, 2021 was $1,161,700.
The fair values of options granted during the three months ended March 31, 2021 were estimated at the date of grant using the Black-Scholes option-pricing model with the following assumptions: volatility of 128.42%, risk-free rate of 0.36%, annual dividend yield of 0% and expected life of 5.00 years. The aggregate fair value of the options granted during the three months ended March 31, 2021 was $419,020.
The fair values of options granted during the three months ended March 31, 2020 were estimated at the date of grant using the Black-Scholes option-pricing model with the following assumptions: volatility of 137.42% - 139.58%, risk-free rate of 1.39% - 1.67%, annual dividend yield of 0% and expected life of 5.00 – 10.00 years. The aggregate fair value of the options granted during the three months ended March 31, 2020 was $2,422,225.
For the three months ended March 31, 2021 and 2020, stock-based compensation expense associated with stock options granted amounted to $202,505 and $785,350, respectively, of which, $139,507 and $674,998 was recorded as compensation and related benefits, $43,443 and $103,818 was recorded as professional fees, and $19,555 and $6,534 was recorded as research and development expenses, respectively.
F-74
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 8 – EQUITY (continued)
Options (continued)
A summary of the status of the Company’s nonvested stock options granted as of March 31, 2021 and changes during the three months ended March 31, 2021 is presented below:
| Number of Options | Weighted Average Exercise Price | |||||||
| Nonvested at January 1, 2021 | 218,334 | $ | 1.18 | |||||
| Granted | 440,000 | 1.12 | ||||||
| Vested | (215,000 | ) | (1.15 | ) | ||||
| Nonvested at March 31, 2021 | 443,334 | $ | 1.14 | |||||
NOTE 9 - STATUTORY RESERVE
Avalon Shanghai and Beijing Genexosome operate in the PRC, are required to reserve 10% of their net profit after income tax, as determined in accordance with the PRC accounting rules and regulations. Appropriation to the statutory reserve by the Company is based on profit arrived at under PRC accounting standards for business enterprises for each year.
The profit arrived at must be set off against any accumulated losses sustained by the Company in prior years, before allocation is made to the statutory reserve. Appropriation to the statutory reserve must be made before distribution of dividends to shareholders. The appropriation is required until the statutory reserve reaches 50% of the registered capital. This statutory reserve is not distributable in the form of cash dividends. The Company did not make any appropriation to statutory reserve for Avalon Shanghai and Beijing Genexosome during the three months ended March 31, 2021 and 2020 as they incurred net losses in these periods.
NOTE 10 – RESTRICTED NET ASSETS
A portion of the Company’s operations are conducted through its PRC subsidiaries, which can only pay dividends out of their retained earnings determined in accordance with the accounting standards and regulations in the PRC and after they have met the PRC requirements for appropriation to statutory reserve. In addition, a portion of the Company’s businesses and assets are denominated in RMB, which is not freely convertible into foreign currencies. All foreign exchange transactions take place either through the People’s Bank of China or other banks authorized to buy and sell foreign currencies at the exchange rates quoted by the People’s Bank of China. Approval of foreign currency payments by the People’s Bank of China or other regulatory institutions requires submitting a payment application form together with suppliers’ invoices, shipping documents and signed contracts. These currency exchange control procedures imposed by the PRC government authorities may restrict the ability of the Company’s PRC subsidiaries to transfer their net assets to the Parent Company through loans, advances or cash dividends.
Schedule I of Article 5-04 of Regulation S-X requires the condensed financial information of the parent company to be filed when the restricted net assets of consolidated subsidiaries exceed 25 percent of consolidated net assets as of the end of the most recently completed fiscal year. For purposes of this test, restricted net assets of consolidated subsidiaries shall mean that amount of the registrant’s proportionate share of net assets of its consolidated subsidiaries (after intercompany eliminations) which as of the end of the most recent fiscal year may not be transferred to the parent company in the form of loans, advances or cash dividends without the consent of a third party.
The Company’s PRC subsidiaries’ net assets as of March 31, 2021 and December 31, 2020 did not exceed 25% of the Company’s consolidated net assets. Accordingly, the Parent Company’s condensed consolidated financial statements have not been required in accordance with Rule 5-04 and Rule 12-04 of SEC Regulation S-X.
NOTE 11 - CONCENTRATIONS
Customers
The following table sets forth information as to each customer that accounted for 10% or more of the Company’s revenues for the three months ended March 31, 2021 and 2020.
| Three Months Ended March 31, | ||||||||
| Customer | 2021 | 2020 | ||||||
| A | 30 | % | 30 | % | ||||
| B | 20 | % | 18 | % | ||||
| C | 13 | % | 15 | % | ||||
| * | Less than 10% |
One customer, whose outstanding receivable accounted for 10% or more of the Company’s total outstanding accounts receivable, accounts receivable – related party, and rent receivable at March 31, 2021, accounted for 70.3% of the Company’s total outstanding accounts receivable, accounts receivable – related party, and rent receivable at March 31, 2021.
F-75
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 11 – CONCENTRATIONS (continued)
Customers (continued)
Two customers, whose outstanding receivable accounted for 10% or more of the Company’s total outstanding accounts receivable, accounts receivable – related party, and rent receivable at December 31, 2020, accounted for 78.3% of the Company’s total outstanding accounts receivable, accounts receivable – related party, and rent receivable at December 31, 2020.
Suppliers
No supplier accounted for 10% or more of the Company’s purchase during the three months ended March 31, 2021 and 2020.
One supplier, whose outstanding payable accounted for 10% or more of the Company’s total outstanding accounts payable at March 31, 2021, accounted for 90.2% of the Company’s total outstanding accounts payable at March 31, 2021.
One supplier, whose outstanding payable accounted for 10% or more of the Company’s total outstanding accounts payable at December 31, 2020, accounted for 93.6% of the Company’s total outstanding accounts payable at December 31, 2020.
NOTE 12 – SEGMENT INFORMATION
For the three months ended March 31, 2020, the Company operated in three reportable business segments - (1) the real property operating segment, (2) the medical related consulting services segment, and (3) the performing development services for hospitals and other customers and sales of developed products to hospitals and other customers segment.
Due to the winding down of the development services and sales of developed products segment in 2020, the Company no longer has any material revenues or expenses in this segment. As a result, commencing from the first quarter of 2021, the Company’s chief operating decision maker no longer reviews development services and sales of developed products operating results.
For the three months ended March 31, 2021, the Company operated in two reportable business segments - (1) the real property operating segment, and (2) the medical related consulting services segment.
The Company’s reportable segments are strategic business units that offer different services and products. They are managed separately based on the fundamental differences in their operations. Information with respect to these reportable business segments for the three months ended March 31, 2021 and 2020 was as follows:
F-76
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 12 – SEGMENT INFORMATION (continued)
| Three Months Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| Revenues | ||||||||
| Real property operations | $ | 289,774 | $ | 296,956 | ||||
| Costs and expenses | ||||||||
| Real property operations | 216,894 | 254,501 | ||||||
| Gross profit | ||||||||
| Real property operations | 72,880 | 42,455 | ||||||
| Other operating expenses | ||||||||
| Real property operations | 101,423 | 110,816 | ||||||
| Medical related consulting services | 161,553 | 155,235 | ||||||
| Development services and sales of developed products | - | 35,999 | ||||||
| Corporate/Other | 2,113,492 | 2,962,597 | ||||||
| Total | 2,376,468 | 3,264,647 | ||||||
| Other income (expense) | ||||||||
| Interest expense | ||||||||
| Corporate/Other | (45,149 | ) | (42,169 | ) | ||||
| Total | (45,149 | ) | (42,169 | ) | ||||
| Other income (expense) | ||||||||
| Real property operations | 104 | (935 | ) | |||||
| Medical related consulting services | (18,486 | ) | (5,487 | ) | ||||
| Development services and sales of developed products | - | 2 | ||||||
| Corporate/Other | 1 | - | ||||||
| Total | (18,381 | ) | (6,420 | ) | ||||
| Total other expense, net | (63,530 | ) | (48,589 | ) | ||||
| Net loss | ||||||||
| Real property operations | 28,439 | 69,296 | ||||||
| Medical related consulting services | 180,039 | 160,722 | ||||||
| Development services and sales of developed products | - | 35,997 | ||||||
| Corporate/Other | 2,158,640 | 3,004,766 | ||||||
| Total | $ | 2,367,118 | $ | 3,270,781 | ||||
| Identifiable long-lived tangible assets at
March 31, 2021 and December 31, 2020 | March 31, 2021 | December 31, 2020 | ||||||
| Real property operations | $ | 7,655,918 | $ | 7,697,473 | ||||
| Medical related consulting services | 208,561 | 223,459 | ||||||
| Development services and sales of developed products | - | 243,869 | ||||||
| Corporate/Other | 220,433 | - | ||||||
| Total | $ | 8,084,912 | $ | 8,164,801 | ||||
F-77
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 12 – SEGMENT INFORMATION (continued)
| Identifiable long-lived tangible assets at
March 31, 2021 and December 31, 2020 | March 31, 2021 | December 31, 2020 | ||||||
| United States | $ | 7,718,174 | $ | 7,764,947 | ||||
| China | 366,738 | 399,854 | ||||||
| Total | $ | 8,084,912 | $ | 8,164,801 | ||||
NOTE 13 – COMMITMENTS AND CONTINGENCIES
Litigation
From time to time, the Company is subject to ordinary routine litigation incidental to its normal business operations. The Company is not currently a party to, and its property is not subject to, any material legal proceedings, except as set forth below.
On October 25, 2017, Genexosome entered into and closed a Stock Purchase Agreement with Beijing Genexosome and Yu Zhou, MD, PhD, the sole shareholder of Beijing Genexosome, pursuant to which Genexosome acquired all of the issued and outstanding securities of Beijing Genexosome in consideration of a cash payment in the amount of $450,000, of which $100,000 is still owed. Further, on October 25, 2017, Genexosome entered into and closed an Asset Purchase Agreement with Dr. Zhou, pursuant to which the Company acquired all assets, including all intellectual property and exosome separation systems, held by Dr. Zhou pertaining to the business of researching, developing and commercializing exosome technologies. In consideration of the assets, Genexosome paid Dr. Zhou $876,087 in cash, transferred 500,000 shares of common stock of the Company to Dr. Zhou and issued Dr. Zhou 400 shares of common stock of Genexosome. Further, the Company had not been able to realize the financial projections provided by Dr. Zhou at the time of the acquisition and has decided to impair the intangible asset associated with this acquisition to zero on September 30, 2019. Dr. Zhou was terminated as Co-CEO of Genexosome on August 14, 2019. Further, on October 28, 2019, Research Institute at Nationwide Children’s Hospital (“Research Institute”) filed a Complaint in the United States District Court for the Southern District of Ohio Eastern Division against Dr. Zhou, Li Chen, the Company and Genexosome with various claims against the Company and Genexosome including misappropriation of trade secrets in violation of the Defend Trade Secrets Act of 2016 and violation of Ohio Uniform Trade Secrets Act. Research Institute is seeking monetary damages, injunctive relief, exemplary damages, injunctive relief and other equitable relief. The Company intends to vigorously defend against this action and pursue all available legal remedies. The civil case against Avalon is stayed pending resolution of the criminal proceedings against Dr. Zhou and Li Chen, and while there can be no assurances, the Company believes it has substantial legal and factual defenses to the Research Institute’s claims and the likelihood of any findings of liability for the Company cannot be assessed at this time.
Operating Leases Commitment
The Company is a party to leases for office space. Rent expense under all operating leases amounted to approximately $39,000 for both the three months ended March 31, 2021 and 2020.
Supplemental cash flow information related to leases for the three months ended March 31, 2021 and 2020 is as follows:
| Three Month Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| Cash paid for amounts included in the measurement of lease liabilities: | ||||||||
| Operating cash flows paid for operating lease | $ | 29,590 | $ | - | ||||
| Right-of-use assets obtained in exchange for lease obligation: | ||||||||
| Operating lease | $ | 133,201 | $ | 185,401 | ||||
The following table summarizes the lease term and discount rate for the Company’s operating lease as of March 31, 2021:
| Operating Lease | ||||
| Weighted average remaining lease term (in years) | 1.83 | |||
| Weighted average discount rate | 4.88 | % | ||
F-78
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 13 – COMMITMENTS AND CONTINCENGIES (continued)
Operating Leases Commitment (continued)
The following table summarizes the maturity of lease liabilities under operating lease as of March 31, 2021:
| For the Twelve-month Period Ending March 31: | Operating Lease | |||
| 2022 | $ | 140,817 | ||
| 2023 | 111,347 | |||
| 2024 and thereafter | - | |||
| Total lease payments | 252,164 | |||
| Amount of lease payments representing interest | (10,170 | ) | ||
| Total present value of operating lease liabilities | $ | 241,994 | ||
| Current portion | $ | 132,657 | ||
| Long-term portion | 109,337 | |||
| Total | $ | 241,994 | ||
Equity Investment Commitment
On May 29, 2018, Avalon Shanghai entered into a Joint Venture Agreement with Jiangsu Unicorn Biological Technology Co., Ltd. (“Unicorn”), pursuant to which a company named Epicon Biotech Co., Ltd. (“Epicon”) was formed on August 14, 2018. Epicon is owned 60% by Unicorn and 40% by Avalon Shanghai. Within five years of execution of the Joint Venture Agreement, Unicorn shall invest cash into Epicon in an amount not less than RMB 8,000,000 (approximately $1.2 million) and the premises of the laboratories of Nanjing Hospital of Chinese Medicine for exclusive use by Epicon, and Avalon Shanghai shall invest cash into Epicon in an amount not less than RMB 10,000,000 (approximately $1.5 million). Epicon is focused on cell preparation, third party testing, biological sample repository for commercial and scientific research purposes and the clinical transformation of scientific achievements. As of March 31, 2021, Avalon Shanghai has contributed RMB 4,700,000 (approximately $0.7 million) that was included in equity method investment on the accompanying condensed consolidated balance sheets. The Company intends to use its present working capital together with borrowings from related party and equity raises to fund the project cost.
Joint Venture – AVAR BioTherapeutics (China) Co. Ltd.
On October 23, 2018, Avactis Biosciences, Inc. (“Avactis”), a wholly-owned subsidiary of the Company, and Arbele Limited (“Arbele”) agreed to the establishment of AVAR BioTherapeutics (China) Co. Ltd. (“AVAR”), a Sino-foreign equity joint venture, pursuant to an Equity Joint Venture Agreement (the “AVAR Agreement”), which will be owned 60% by Avactis and 40% by Arbele. The purpose and business scope of the Joint Venture is to research, develop, produce, sell, distribute and generally commercialize CAR-T/CAR-NK/TCR-T/universal cellular immunotherapy in China. Avactis is required to contribute $10 million (or equivalent in RMB) in cash and/or services, which shall be contributed in tranches based on milestones to be determined jointly by AVAR and Avactis in writing subject to Avactis’ cash reserves. Within 30 days, Arbele shall make a contribution of $6.66 million in the form of entering into a License Agreement with AVAR granting AVAR with an exclusive right and license in China to its technology and intellectual property pertaining to CAR-T/CAR-NK/TCR-T/universal cellular immunotherapy technology and any additional technology developed in the future with terms and conditions to be mutually agreed upon Avactis and AVAR and services.
F-79
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 13 – COMMITMENTS AND CONTINGENCIES (continued)
Joint Venture – AVAR BioTherapeutics (China) Co. Ltd. (continued)
In addition, Avactis is responsible for:
| ● | Contributing registered capital of RMB 5,000,000 (approximately $0.8 million) for working capital purposes as required by local regulation, which is not required to be contributed immediately and will be contributed subject to Avactis’ discretion; |
| ● | assist AVAR in setting up its business operations and obtaining all required permits and licenses from the Chinese government; |
| ● | assisting AVAR in recruiting, hiring and retaining personnel; |
| ● | providing AVAR with access to various hospital networks in China to assist in the testing and commercialization of the CAR-T/CAR-NK/TCR-T/universal cellular immunotherapy technology in China; |
| ● | assisting AVAR in managing the Good Manufacturing Practices (GMP) facility and clinic to be developed by AVAR; |
| ● | providing AVAR with advice pertaining to conducting clinicals in China; and |
| ● | Within 6 days of signing the AVAR Agreement, Avactis is required to pay to Arbele $300,000 as a research and development fee with an additional two payments of $300,000 (for a total of $900,000) to be paid upon mutually agreed upon milestones. |
Under AVAR Agreement, Arbele shall be responsible for the following:
| ● | Entering into a License Agreement with AVAR; and | |
| ● | Providing AVAR with research and development expertise pertaining to clinical laboratory medicine when hired by AVAR. |
As of March 31, 2021 and December 31, 2020, Avactis has paid $900,000 to Arbele as research and development fee. As of March 31, 2021, License Agreement has not been finalized.
Line of Credit Agreement
On August 29, 2019, the Company entered into a Line of Credit Agreement (the “Line of Credit Agreement”) providing the Company with a $20 million line of credit (the “Line of Credit”) from Wenzhao Lu (the “Lender”), a significant shareholder and director of the Company. The Line of Credit allows the Company to request loans thereunder and to use the proceeds of such loans for working capital and operating expense purposes until the facility matures on December 31, 2024. The loans are unsecured and are not convertible into equity of the Company. Loans drawn under the Line of Credit bears interest at an annual rate of 5% and each individual loan will be payable three years from the date of issuance. The Company has a right to draw down on the line of credit and not at the discretion of the related party Lender. The Company may, at its option, prepay any borrowings under the Line of Credit, in whole or in part at any time prior to maturity, without premium or penalty. The Line of Credit Agreement includes customary events of default. If any such event of default occurs, the Lender may declare all outstanding loans under the Line of Credit to be due and payable immediately. As of March 31, 2021, $3,305,249 was outstanding under the Line of Credit.
NOTE 14 – SUBSEQUENT EVENTS
Management has evaluated subsequent events through the date of the filing.
F-80
Purchase Agreement
SHARE PURCHASE AGREEMENT
by and among
AVALON GLOBOCARE CORP.
THE SEN LANG SHAREHOLDERS,
THE SEN LANG BENEFICIAL SHAREHOLDERS,
SEN LANG BVI,
AND
THE SEN LANG REPRESENTATIVE
June 13, 2021
Table of contents
| ARTICLE I. PURCHASE AND SALE OF SEN LANG SHARES | A-2 | |
| 1.1 | Closing Date | A-2 |
| 1.2 | The Acquisition | A-2 |
| 1.3 | Shareholder Consideration | A-3 |
| ARTICLE II. CONVERSION AND DISTRIBUTION OF SECURITIES | A-3 | |
| 2.1 | Distributions; Exchange Ratio; Fractional Shares | A-3 |
| 2.2 | Exchange of Certificates | A-3 |
| 2.3 | Escrow | A-4 |
| 2.4 | Withholding | A-5 |
| 2.5 | Guaranty | A-5 |
| ARTICLE III. REPRESENTATIONS AND WARRANTIES OF THE SEN LANG OWNERS | A-5 | |
| 3.1 | Organization and Qualification | A-5 |
| 3.2 | Authority Relative to this Agreement | A-6 |
| 3.3 | Consents, No Conflicts | A-7 |
| 3.4 | Board Recommendation | A-7 |
| 3.5 | Stockholder Protection Rights Agreements | A-7 |
| 3.6 | No Existing Violation, Default, Etc | A-8 |
| 3.7 | Licenses and Permits | A-8 |
| 3.8 | Proxy Statement | A-8 |
| 3.9 | Finders or Brokers; Compensation Arrangements | A-9 |
| 3.10 | Financial Statements | A-9 |
| 3.11 | Undisclosed Liabilities | A-9 |
| 3.12 | Absence of Changes or Events | A-9 |
| 3.13 | Capitalization | A-10 |
| 3.14 | Capital Stock of Subsidiaries | A-10 |
| 3.15 | Litigation | A-11 |
| 3.16 | Insurance | A-11 |
| 3.17 | Title to and Condition of Properties | A-11 |
| 3.18 | Leases | A-12 |
| 3.19 | Contracts and Commitments | A-12 |
| 3.20 | Employees; Labor Matters | A-13 |
| 3.21 | No Change of Control Puts | A-14 |
| 3.22 | Employment and Labor Contracts | A-14 |
| 3.23 | Intellectual Property Rights | A-14 |
| 3.24 | Taxes | A-15 |
| 3.25 | Employee Benefit Plans | A-18 |
A-i
| 3.26 | Environmental Matters | A-18 |
| 3.27 | [INTENTIONALLY OMITTED.] | A-19 |
| 3.28 | Security Holders’ Identity and Compliance | A-19 |
| 3.29 | Other Representations and Warranties Relating to the PRC Companies | A-19 |
| 3.30 | Captive Structure | A-20 |
| 3.31 | Foreign Government Ownership Under CFIUS | A-21 |
| 3.32 | No Sanctioned Persons | A-21 |
| 3.33 | Disclosure | A-21 |
| ARTICLE IV. REPRESENTATIONS AND WARRANTIES OF AVALON | A-21 | |
| 4.1 | Organization and Qualification | A-21 |
| 4.2 | Authority Relative to this Agreement | A-22 |
| 4.3 | Consents, No Conflicts | A-22 |
| 4.4 | Board Recommendation | A-23 |
| 4.5 | Stockholder Protection Rights Agreements | A-23 |
| 4.6 | No Existing Violation, Default, Etc | A-24 |
| 4.7 | Licenses and Permits | A-24 |
| 4.8 | Proxy Statement | A-24 |
| 4.9 | Finders or Brokers; Compensation Arrangements | A-25 |
| 4.10 | SEC Reports | A-25 |
| 4.11 | Disclosure Controls and Procedures | A-25 |
| 4.12 | Financial Statements | A-25 |
| 4.13 | SOX Certifications | A-26 |
| 4.14 | Undisclosed Liabilities | A-26 |
| 4.15 | Off-Balance Sheet Arrangements | A-26 |
| 4.16 | Loans to Executives and Directors | A-26 |
| 4.17 | Independent Auditors | A-26 |
| 4.18 | Absence of Changes or Events | A-27 |
| 4.19 | Capitalization | A-27 |
| 4.20 | Capital Stock of Subsidiaries | A-28 |
| 4.21 | Litigation | A-28 |
| 4.22 | Insurance | A-29 |
| 4.23 | Title to and Condition of Properties | A-29 |
| 4.24 | Leases | A-30 |
| 4.25 | Contracts and Commitments | A-30 |
| 4.26 | Employees; Labor Matters | A-31 |
| 4.27 | Put Rights | A-31 |
| 4.28 | Employment and Labor Contracts | A-31 |
| 4.29 | Intellectual Property Rights | A-32 |
| 4.30 | Taxes | A-32 |
| 4.31 | Employee Benefit Plans | A-33 |
| 4.32 | Environmental Matters | A-33 |
A-ii
| ARTICLE V. COVENANTS OF THE PARTIES | A-34 | |
| 5.1 | Access and Information | A-34 |
| 5.2 | Sen Lang’s Affirmative Covenants | A-35 |
| 5.3 | Avalon’s Affirmative Covenants | A-36 |
| 5.4 | Sen Lang’s Negative Covenants | A-37 |
| 5.5 | Avalon’s Negative Covenants | A-38 |
| 5.6 | Exclusive Dealing | A-39 |
| 5.7 | Closing Documents | A-39 |
| 5.8 | Further Actions | A-40 |
| 5.9 | Public Announcements | A-40 |
| 5.10 | Stockholders’ Meetings | A-40 |
| 5.11 | Preparation of the Proxy Statement | A-41 |
| 5.12 | Nasdaq Listing | A-41 |
| 5.13 | Financial Statements for a Current Report on Form 8-K | A-41 |
| 5.14 | Business of the PRC Companies | A-42 |
| 5.15 | Control Documents | A-42 |
| 5.16 | Circular 37 | A-43 |
| 5.17 | Human Genetic Resources | A-43 |
| 5.18 | PRC Tax Circular 7 | A-43 |
| 5.19 | Rule 144 | A-44 |
| ARTICLE VI. CONDITIONS | A-45 | |
| 6.1 | Conditions to the Obligations of Each Party | A-45 |
| 6.2 | Conditions to Avalon’s Obligations | A-45 |
| 6.3 | Conditions to Sen Lang’s Obligations | A-48 |
| ARTICLE VII. TERMINATION | A-49 | |
| 7.1 | Termination | A-49 |
| 7.2 | Effect of Termination | A-51 |
| ARTICLE VIII. SURVIVAL AND INDEMNIFICATION | A-51 | |
| 8.1 | Survival of Representations and Warranties | A-51 |
| 8.2 | Limitations and General Indemnification Provisions | A-53 |
| 8.3 | Indemnification Procedures | A-53 |
| 8.4 | Indemnification Payments | A-55 |
| 8.5 | Exclusive Remedy | A-55 |
| ARTICLE IX. MISCELLANEOUS | A-56 | |
| 9.1 | Amendments, Extensions and Waivers | A-56 |
| 9.2 | Notices | A-57 |
| 9.3 | Interpretation; Construction | A-57 |
| 9.4 | “Knowledge” Defined | A-57 |
| 9.5 | Counterparts | A-57 |
| 9.6 | Entire Agreement | A-57 |
| 9.7 | Third-Party Beneficiaries | A-57 |
A-iii
| 9.8 | Governing Law | A-57 |
| 9.9 | Consent to Jurisdiction; Venue; Waiver of Jury Trial | A-58 |
| 9.10 | Specific Performance | A-58 |
| 9.11 | Assignment | A-59 |
| 9.12 | Expenses | A-59 |
| 9.13 | Severability | A-59 |
| 9.14 | Release | A-59 |
| 9.15 | Governing Language; Translations | A-60 |
| 9.16 | Voluntary Agreement | A-60 |
| ARTICLE X. DEFINITIONS | A-60 | |
| 10.1 | Certain Definitions | A-60 |
A-iv
SHARE PURCHASE AGREEMENT
This Share Purchase Agreement (this “Agreement”) is made and entered into as of the 13th day of June, 2021 (the “Effective Date”), by and among Avalon GloboCare Corp., a Delaware corporation (“Avalon”), Lonlon Biotech Ltd., a company incorporated in the British Virgin Islands (“BVI”) (“Sen Lang”), the holders of the Sen Lang Shares (as defined herein), as set forth on Exhibit A hereto (each individually, a “Sen Lang Shareholder” and collectively, the “Sen Lang Shareholders”), the beneficial owner of each Sen Lang Shareholder, as set forth on Exhibit A hereto (each individually, a “Sen Lang Beneficial Shareholder” and collectively, the “Sen Lang Beneficial Shareholders”, and together with the Sen Lang Shareholders, the “Sen Lang Owners”), and Ding Wei, in the capacity as the representative from and after the Closing for the Sen Lang Owners as of immediately prior to the Closing in accordance with the terms and conditions of this Agreement (the “Sen Lang Representative”). Avalon, Sen Lang, the Sen Lang Shareholders, the Sen Lang Beneficial Shareholders and the Sen Lang Representative are sometimes collectively referred to as the “Parties”, and each, individually, as a “Party”. Sen Lang, the Sen Lang Shareholders, the Sen Lang Beneficial Shareholders and the Sen Lang Representative are sometimes collectively referred to as the “Sen Lang Parties”, and each, individually, as a “Sen Lang Party”.
PRELIMINARY STATEMENTS
A. Avalon is a clinical-stage, bio-developer of innovative and transformative cell-based technologies and their clinical applications.
B. Lonlon Biotech Investment Limited is a company with limited liability organized and existing under the laws of Hong Kong Special Administrative Region (“Hong Kong”) (the “HK Subsidiary”). The HK Subsidiary is a wholly owned enterprise of Sen Lang and Sen Lang owns 100% of the equity interest in the HK Subsidiary.
C. Beijing Langlang Runfeng Biotechnology Co., Ltd. (北京朗朗润丰生物科技有限公司 in Chinese) is a wholly foreign owned enterprise with limited liability organized and existing under the laws of the People’s Republic of China (the “PRC”, for the purpose of this Agreement, excluding Hong Kong, the Macau Special Administrative Region and Taiwan) (the “PRC Subsidiary”). The PRC Subsidiary is a wholly owned enterprise of the HK Subsidiary and the HK Subsidiary owns 100% of the equity interest in the PRC Subsidiary.
D. Senlang Biotechnology Co. Ltd. (河北森朗生物科技有限公司 in Chinese) is a PRC domestic company with limited liability organized and existing under the laws of the PRC (the “OpCo”). The OpCo is mainly engaged in the business of the research and development in relation to CAR-T cell therapy, immune cell therapy and related drug (the “Principal Business”). 13 Sen Lang Beneficial Shareholders out of the total 15 Sen Lang Beneficial Shareholders own the aggregate 100% equity interests in the OpCo.
E. Shijiazhuang Senlang Medical Laboratory Co., Ltd. (石家庄森朗医学检验实验室有限公司 in Chinese) is a PRC domestic company with limited liability organized and existing under the laws of the PRC (the “Senlang Lab”). The Senlang Lab is mainly engaged in the business of testing of immunology, serology and molecular genetics specialties for patients, including hematology-tumor diagnostics and testing prior to clinical trials for cell therapy. Senlang Lab is a wholly owned enterprise of OpCo and OpCo owns 100% of the equity interest in Senlang Lab. The PRC Subsidiary, the OpCo and Senlang Lab are collectively referred to as the “PRC Companies”.
A-1
G. Avalon desires to acquire Sen Lang through the acquisition of all of the issued and outstanding share capital (the “Sen Lang Shares”) of Sen Lang from the Sen Lang Shareholders (the “Acquisition”). Each of the Parties has determined that the Acquisition is consistent with and in furtherance of its respective long-term business strategies and desires to combine their respective businesses and for the Sen Lang Shareholders to have a continuing equity interest in the combined Avalon/Sen Lang businesses through the ownership of shares of common stock, par value $0.0001 per share, of Avalon (the “Avalon Common Stock”).
H. Pursuant to the terms and subject to the conditions set forth in this Agreement as consideration in the Acquisition, Avalon shall issue shares of Avalon Common Stock to each Sen Lang Shareholder.
I. The respective Boards of Directors of Avalon and Sen Lang have determined that the Acquisition, in the manner contemplated herein, is desirable and in the best interests of their respective shareholders and stockholders and, by resolutions duly adopted, have approved and adopted this Agreement.
J. Simultaneously with the execution and delivery of this Agreement, the Sen Lang Owners have each delivered an Accredited Investor Questionnaire & Representation Letter with Avalon, substantially in the form attached hereto as Exhibit B (each, an “AI Letter”).
NOW, THEREFORE, in consideration of these premises and the mutual and dependent promises hereinafter set forth, the parties hereto hereby agree as follows:
ARTICLE I.
PURCHASE AND SALE OF SEN LANG SHARES
1.1 Closing Date. The closing of the Acquisition (the “Closing”) shall take place remotely via the electronic exchange of documents and signatures on the third (3rd) Business Day after the satisfaction (or waiver) of the conditions set forth in ARTICLE VI (not including conditions which are to be satisfied by actions taken at the Closing, but subject to the satisfaction of such conditions on the Closing Date or waiver by the party entitled to waive such conditions), unless another time, date or place is agreed to in writing by the Sen Lang Representative and Avalon. The “Closing Date” shall be the date on which the Closing is consummated.
1.2 The Acquisition. On the terms and subject to the conditions hereof, on the Closing Date, (a) each Sen Lang Shareholder shall sell, assign, transfer and convey to Avalon, and Avalon shall purchase and acquire from each such Sen Lang Shareholder, all of the Sen Lang Shares owned by such Sen Lang Shareholder, free and clear of any Liens and restrictions on transfer. During the Executory Period, no Sen Lang Shareholder shall sell, assign, transfer, convey or otherwise dispose of any Sen Lang Shares held by such Sen Lang Shareholder to any other Person.
A-2
1.3 Shareholder Consideration.
1.3.1 As consideration for the Acquisition, the Sen Lang Shareholders collectively shall be entitled to receive from Avalon, in the aggregate, 81,000,000 shares of Avalon Common Stock.
1.3.2 The 81,000,000 shares of Avalon Common Stock to be issued to the Sen Lang Shareholders is referred to as the “Common Exchange Shares”; the number of Common Exchange Shares received by each Sen Lang Shareholder is referred to as the “Exchange Stocks”; provided, that the Common Exchange Shares otherwise payable to the Sen Lang Shareholders at the Closing is subject to the withholding of the Escrow Shares deposited in the Escrow Account in accordance with Section 2.3, and after the Closing is subject to reduction for the indemnification obligations of the Indemnifying Parties set forth in ARTICLE VIII.
ARTICLE II.
CONVERSION AND DISTRIBUTION OF SECURITIES
2.1 Distributions; Exchange Ratio; Fractional Shares.
2.1.1 As soon as practical after the effectiveness of the Acquisition, each Sen Lang Shareholder shall receive, for each Sen Lang Share held by such Sen Lang Shareholder, a number of shares of Avalon Common Stock equal to the Exchange Stocks of such Sen Lang Shareholder set forth on Exhibit A hereto.
2.1.2 No certificates for fractional shares of Avalon Common Stock shall be issued as a result of the distribution provided for in this Section 2.1. In lieu of any fractional share to which the Sen Lang Shareholders would otherwise be entitled as a result of the distribution provided for in Section 2.1, all issuances of Avalon Common Stock shall be rounded to the nearest whole share.
2.1.3 In the event that, subsequent to the date hereof and prior to the Closing, Avalon shall declare a stock dividend or other distribution payable in shares of Avalon Common Stock or securities convertible into shares of Avalon Common Stock or effect a stock split, reclassification, combination or other change with respect to shares of Avalon Common Stock, the number of shares of Avalon Common Stock set forth in Section 1.3.1 and the Exchange Stocks of each Sen Lang Shareholder set forth on Exhibit A hereto shall be adjusted to reflect such dividend, distribution, stock split, reclassification, combination or other change.
2.2 Exchange of Certificates.
2.2.1 Exchange Agent. Promptly following the Closing, Avalon shall deposit with VStock Transfer, LLC or such other exchange agent as may be designated by Avalon (the “Exchange Agent”), for the benefit of Sen Lang Shareholders, for distribution in accordance with this Section 2.2, certificates representing the Common Exchange Shares, subject to reduction for the Escrow Shares pursuant to Section 2.3, for distribution to holders of the Sen Lang Shares pursuant to Section 2.1.2 (such shares of Avalon Common Stock being hereinafter also referred to as the “Exchange Fund”).
A-3
2.2.2 Exchange Procedures.
2.2.2.1 At or prior to the Closing, Avalon shall send, or shall cause the Exchange Agent to send, to each Sen Lang Shareholder, a letter of transmittal for use in such exchange, substantially in the form attached hereto as Exhibit C (a “Letter of Transmittal”) (which shall specify that the delivery of Sen Lang Certificates (as defined herein) in respect of the Common Exchange Shares shall be effected, and risk of loss and title shall pass, only upon proper delivery of the Sen Lang Certificates to the Exchange Agent (or a Lost Certificate Affidavit) (as defined herein)) for use in such exchange.
2.2.2.2 Each Sen Lang Shareholder shall be entitled to receive its portion of the Common Exchange Shares as set forth on Exhibit A hereto (less the Escrow Shares) in respect of the Sen Lang Shares represented by the certificates representing the Sen Lang Shares (the “Sen Lang Certificates”), as soon as reasonably practicable after the Closing, but subject to the delivery to the Exchange Agent of the following items prior thereto (collectively, the “Transmittal Documents”): (i) the Sen Lang Certificate(s) for its Sen Lang Shares (or a Lost Certificate Affidavit), together with a properly completed and duly executed Letter of Transmittal, (ii) a properly completed and duly executed AI Letter, and (iii) such other documents as may be reasonably requested by the Exchange Agent or Avalon. Until so surrendered, each Sen Lang Certificate shall represent after the Closing for all purposes only the right to receive such portion of the Common Exchange Shares (subject to the withholding of the Escrow Shares) attributable to such Sen Lang Certificate.
2.3 Escrow.
2.3.1 At or prior to the Closing, Avalon, the Sen Lang Representative and VStock Transfer, LLC (or such other escrow agent mutually acceptable to Avalon and the Sen Lang Representative), as escrow agent (the “Escrow Agent”), shall enter into an escrow agreement, effective as of the Closing, substantially in the form attached hereto as Exhibit D (the “Escrow Agreement”), pursuant to which Avalon shall issue to the Escrow Agent a number of shares of Avalon Common Stock equal to ten percent (10.00%) of the Common Exchange Shares (the “Escrow Amount”) (together with any equity securities paid as dividends or distributions with respect to such shares or into which such shares are exchanged or converted, the “Escrow Shares”) to be held, along with any other dividends, distributions or other income on the Escrow Shares (together with the Escrow Shares, the “Escrow Property”), in a segregated escrow account (the “Escrow Account”) and disbursed therefrom in accordance with the terms of ARTICLE VIII hereof and the Escrow Agreement. The Escrow Property shall be allocated among and transferred to the Sen Lang Shareholders pro rata based on their respective Pro Rata Share. The Escrow Property shall not serve as the sole source of payment for the obligations of the Sen Lang Shareholders pursuant to ARTICLE VIII. Unless otherwise required by law, all distributions made from the Escrow Account shall be treated by the Parties as an adjustment to the number of shares of Common Exchange Shares received by the Sen Lang Shareholders pursuant to this ARTICLE II.
A-4
2.3.2 The Escrow Property shall be released from escrow on the date which is twelve (12) months after the Closing Date (the “Escrow Release Date”); provided, however, with respect to any indemnification claims made in accordance with ARTICLE VIII hereof on or prior to the Escrow Release Date that remain unresolved at the time of the Escrow Release Date (“Pending Claims”), all or a portion of the Escrow Property reasonably necessary to satisfy such Pending Claims (as determined based on the amount of the indemnification claim included in the Claim Notice provided by Avalon under ARTICLE VIII and the Avalon Per Share Price) shall remain in the Escrow Account until such time as such Pending Claim shall have been finally resolved and paid pursuant to the provisions of ARTICLE VIII. After the Escrow Release Date, any Escrow Property remaining in the Escrow Account that is not subject to Pending Claims, if any, and not subject to resolved but unpaid claims in favor of an Indemnified Party, shall be transferred by the Escrow Agent to the Sen Lang Shareholders that have previously delivered the Transmittal Documents in accordance with Section 2.2.2.2, with each such Sen Lang Shareholder receiving its Pro Rata Share of such Escrow Property. Promptly after the final resolution of all Pending Claims and payment of all indemnification obligations in connection therewith, the Escrow Agent shall transfer any remaining Escrow Property remaining in the Escrow Account to the Sen Lang Shareholders that have previously delivered the Transmittal Documents in accordance with Section 2.2.2.2, with each such Sen Lang Shareholder receiving its Pro Rata Share of such Escrow Property.
2.4 Withholding. Each of Avalon, the Exhange Agent and the Escrow Agent shall be entitled to deduct and withhold from any consideration deliverable pursuant to this Agreement to any Sen Lang Shareholder such amounts as are required to be deducted or withheld from such consideration under the Code or under any other Applicable Law. To the extent such amounts are so deducted or withheld, such amounts shall be treated for all purposes under this Agreement as having been paid to the Person to whom such amounts would otherwise have been paid.
2.5 Guaranty. Notwithstanding any other provision of this Agreement, each Sen Lang Beneficial Shareholder hereby guaranties all obligations under this Agreement of such Sen Lang Shareholder set forth opposite such Sen Lang Beneficial Shareholders’s name on Exhibit A hereto, including the prompt payment of all amounts due to Avalon thereunder by such Sen Lang Shareholder upon demand if not paid when due by such Sen Lang Shareholder.
ARTICLE III.
REPRESENTATIONS AND WARRANTIES OF THE SEN LANG OWNERS
Except as set forth in the Disclosure Schedule delivered by the Sen Lang Owners to Avalon at or prior to the execution of this Agreement (the “Sen Lang Disclosure Schedule”) (each section of which qualifies the correspondingly numbered representation and warranty, regardless of whether such representation or warranty expressly refers to or is qualified by reference to such Sen Lang Disclosure Schedule), the Sen Lang Owners, jointly and severally, represent and warrant, on behalf of the Acquired Companies, to Avalon as follows:
3.1 Organization and Qualification.
3.1.1 Each of the Acquired Companies is an entity duly incorporated, validly existing and in good standing under the laws of the jurisdiction of its incorporation or organization and has the corporate power and authority to own, lease and operate its properties and to conduct its business as currently conducted. Each of the Acquired Companies is duly qualified to transact business as a foreign corporation or other foreign entity and is in good standing in each jurisdiction in which the conduct of its business or the ownership, leasing or operation of its property requires such qualification.
A-5
3.1.2 None of the Acquired Companies is in violation of any of the provisions of its certificate of incorporation or by-laws, or other similar organizational documents, each as amended and currently in effect, or, if it is a limited liability company or partnership, its operating agreement, partnership agreement or other comparable agreement. True and complete copies of the certificate of incorporation and by-laws, each as amended and as currently in effect, of Sen Lang, and true and complete copies of the certificate of incorporation and by-laws, or other similar organizational documents, each as amended and currently in effect, of each Acquired Company have been previously delivered or made available to Avalon.
3.1.3 Sen Lang has full power and authority (corporate and other) and all consents, approvals, authorizations, orders, registrations, clearances and qualifications of or with any Governmental Authority having jurisdiction over Sen Lang or any Acquired Company or any of its or their respective properties required for the ownership and the conduct of its business and has the legal right and authority to own, use, lease and operate its assets and to conduct its business. All of the issued Shares of Sen Lang has been duly and validly authorized and issued and are fully paid and non-assessable. Sen Lang has obtained all approvals, authorizations, consents and orders, and has made all filings and registrations, which are required under BVI laws and regulations for the ownership interest by Avalon of its equity interest in Sen Lang, and there are no outstanding rights, warrants or options to acquire, or instruments convertible into or exchangeable for, nor any agreements or other obligations to issue or other rights to convert any obligation into, any equity interest in Sen Lang.
3.2 Authority Relative to this Agreement. Sen Lang has the corporate power and authority to execute and deliver this Agreement and, upon obtaining the approval of a majority of the outstanding Sen Lang Shares at the Sen Lang Special Meeting (as defined herein) or any adjournment thereof as authorized under the laws of the British Virgin Islands, including the BVI Business Companies Act, 2004, as amended (“BVI Law”), to consummate the Acquisition and the other transactions contemplated hereby. The execution and delivery of this Agreement and the consummation of the Acquisition and the other transactions contemplated hereby have been duly and validly authorized by the Board of Directors of Sen Lang, and except as stated in the preceding sentence, no other corporate proceedings on the part of Sen Lang are necessary to authorize this Agreement or to consummate the Acquisition and the other transactions contemplated hereby. This Agreement has been duly and validly executed and delivered by Sen Lang, and, assuming the due authorization, execution and delivery hereof by Avalon and subject to stockholder approval as aforesaid, constitutes a valid and binding agreement of Sen Lang enforceable against Sen Lang and each Acquired Company in accordance with its terms, except to the extent that its enforceability may be limited by applicable bankruptcy, insolvency, reorganization, moratorium or other laws affecting the enforcement of creditors’ rights generally or by general equitable principles.
A-6
3.3 Consents, No Conflicts.
3.3.1 Except for actions to be taken in connection with (a) filings required pursuant to any state securities or “blue sky” laws, (b) any filings required with the Nasdaq Capital Market (“Nasdaq”) or the SEC with respect to the transactions contemplated by this Agreement, and (c) any other filings, notices, disclosures or registrations set forth in Section 3.3.1 of the Sen Lang Disclosure Schedule, no filing or registration with, notification or disclosure to, or permit, authorization, consent or approval of, (x) any Governmental Authority or (y) any third party, whether acting in an individual, fiduciary or other capacity, is required for the consummation by Sen Lang of the Acquisition or the other transactions contemplated hereby.
3.3.2 Except as set forth in Section 3.3.2 of the Sen Lang Disclosure Schedule, the execution, delivery and performance of this Agreement and the consummation of the Acquisition and the other transactions contemplated hereby and compliance by Sen Lang and the Acquired Companies with any of the provisions hereof do not and will not: (i) subject to obtaining the approval of the Acquisition by the Sen Lang Shareholders, conflict with or result in any breach or violation of any provision of the certificate of incorporation or by-laws, or other similar organizational documents, each as amended, of Sen Lang or any Acquired Company or (ii) result in (1) a breach or violation of, a default under or an event triggering any payment, obligation or acceleration of any obligation pursuant to Sen Lang Employee Benefit Plan (as defined herein) or any grant or award made under any of the foregoing, (2) a breach or violation of, a default under or an event triggering a right of termination of, a default under, or the acceleration of any obligation or the creation of a lien, pledge, security interest or other encumbrance on assets (with or without the giving of notice or the lapse of time or both) pursuant to any provision of, any agreement, license, lease of real or personal property, marketing agreement, contract, note, mortgage, indenture or other obligation of any Acquired Company (“Sen Lang Contracts”) or, subject to making all filings, notifications and disclosures and receipt of all permits, authorizations, consents and approvals referred to in clauses “a” through “c” of Section 3.3.1 or in Section 3.3.1 of the Sen Lang Disclosure Schedule, any law, rule, ordinance or regulation or judgment, decree, order or award to which any Acquired Company is subject or any governmental or non-governmental authorization, consent, approval, registration, franchise, license or permit under which any Acquired Company conducts any of its business, or (3) any other change in the rights or obligations of any party under any of the Sen Lang Contracts, except, with respect to this clause (ii), for breaches, violations, defaults, triggering events, creations of Encumbrances on assets, or changes in rights or obligations which would not, singly or in the aggregate with all other such matters, have a Sen Lang Material Adverse Effect.
3.4 Board Recommendation. The Board of Directors of Sen Lang has, by unanimous written consent, approved and adopted this Agreement, the Acquisition and the other transactions contemplated hereby. At such meeting, the Board of Directors of Sen Lang determined that the consideration to be received by the Sen Lang Shareholders pursuant to the Acquisition is fair to the Sen Lang Shareholders, and recommended that the Sen Lang Shareholders approve and adopt this Agreement, the Acquisition and the other transactions contemplated hereby (the “Sen Lang Board Recommendation”).
3.5 Stockholder Protection Rights Agreements. By virtue of resolutions heretofore approved by Sen Lang’s Boards of Directors, the Acquisition, this Agreement, and the transactions contemplated hereby will not be subject to the restrictions on business combinations with interested stockholders otherwise applicable to the Acquisition, this Agreement, or the transactions contemplated hereby under BVI Law. Sen Lang’s Board of Directors have taken such actions and votes as are necessary on its part to render the provisions of any applicable takeover statutes applicable to any Acquired Company inapplicable to this Agreement, the Acquisition, and the transactions contemplated hereby and thereby. No Acquired Company is a party to any stockholder protection rights agreement or any agreement similar thereto.
A-7
3.6 No Existing Violation, Default, Etc. No Acquired Company is in violation of (A) Applicable Law or (B) any order, decree or judgment of any Governmental Authority having jurisdiction over any Acquired Company. No event of default or event that, but for the giving of notice or the lapse of time or both, would constitute an event of default, exists under any Sen Lang Contract or any lease, permit, license or other agreement or instrument to which any Acquired Company is a party or by which any of them is bound or to which any of the properties, assets or operations of any Acquired Company is subject.
3.7 Licenses and Permits. Each Acquired Company has such certificates, permits, licenses, franchises, consents, approvals, orders, authorizations and clearances from appropriate governmental agencies and bodies (“Sen Lang Licenses”) as are necessary to own, lease or operate its properties and to conduct its business as presently conducted and all such Sen Lang Licenses are valid and in full force and effect, other than any failure to have any such Sen Lang License or any failure of any such Sen Lang License to be valid and in full force and effect as would not, singly or in the aggregate with all such other failures, have a Sen Lang Material Adverse Effect. Each Acquired Company is and, within the period of all applicable statutes of limitations, has been in compliance with its obligations under such Sen Lang Licenses and no event has occurred that allows, or after notice or lapse of time would allow, revocation or termination of such Sen Lang Licenses. No Acquired Company has any knowledge of any facts or circumstances that could reasonably be expected to result in an inability of any Acquired Company to renew any material Sen Lang License. Subject to making all filings, notifications and disclosures and receipt of all permits, authorizations, consents and approvals referred to in Section 3.3.1 of the Sen Lang Disclosure Schedule, neither the execution nor delivery by Sen Lang of this Agreement nor the consummation of any of the transactions contemplated herein will result in any revocation or termination of any material Sen Lang License.
3.8 Proxy Statement. None of the information supplied or to be supplied by Sen Lang or any Acquired Company for inclusion in, and none of the information regarding the Acquired Companies incorporated by reference in, the proxy statement to be sent to the stockholders of Avalon and the Sen Lang Shareholders in connection with the annual meeting of stockholders of Avalon at which such stockholders will be asked to approve the issuance of Avalon Common Stock pursuant to the Acquisition (the “Avalon Annual Meeting”) and the special meeting of the Sen Lang Shareholders at which the Sen Lang Shareholders will be asked to approve the Acquisition and this Agreement (the “Sen Lang Special Meeting”) (such proxy statement, as amended by any amendments thereto, being referred to herein as the “Proxy Statement”), including all amendments and supplements to the Proxy Statement, shall, in the case of the Proxy Statement, on the date or dates the Proxy Statement is first mailed to stockholders of Avalon and the Sen Lang Shareholders and on the date or dates of the Avalon Annual Meeting and the Sen Lang Special Meeting, contain any untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary in order to make the statements therein, in the light of the circumstances under which they were made, not misleading. Sen Lang and the Acquired Companies will supply Avalon with all business, financial, legal, management and other information required for inclusion in a proxy statement under SEC rules.
A-8
3.9 Finders or Brokers; Compensation Arrangements. Neither Sen Lang nor any Acquired Company has employed any investment banker, broker, finder or intermediary in connection with the transactions contemplated hereby who might be entitled to a fee or any commission the receipt of which is conditioned in whole or part upon consummation of the Acquisition.
3.10 Financial Statements. The consolidated balance sheets and the related consolidated statements of income and cash flows (including the related notes thereto) of the Acquired Companies set forth in Section 3.10 of the Sen Lang Disclosure Schedule (collectively, the “Sen Lang Financial Statements”), as of their respective dates, complied in all material respects with applicable accounting requirements and the published rules and regulations with respect thereto, were prepared in accordance with generally accepted accounting principles (except as otherwise noted therein), and present fairly in all material respects, the consolidated financial position of the Acquired Companies, as of their respective dates, and the consolidated results of their operations and their cash flows for the periods presented therein (subject, in the case of the unaudited interim financial statements, to notes and normal year-end adjustments that were not material in amount or effect).
3.11 Undisclosed Liabilities. Except (i) as reflected in Sen Lang’s unaudited consolidated balance sheet at March 31, 2021 or liabilities described in any notes thereto, (ii) for liabilities incurred in the ordinary course of business since March 31, 2021 consistent with past practice or in connection with this Agreement or the transactions contemplated hereby, or (iii) performance obligations under contracts required in accordance with their terms, or performance obligations, to the extent required under Applicable Law, in each case to the extent arising after the date hereof, no Acquired Company has any material liabilities or obligations of any nature (whether accrued, absolute, contingent or otherwise) and which, individually or in the aggregate, could reasonably be expected to have a Sen Lang Material Adverse Effect.
3.12 Absence of Changes or Events. Except for matters disclosed in Section 3.12 of the Sen Lang Disclosure Schedule:
3.12.1 Since December 31, 2018: (i) the Acquired Companies have conducted their business in the ordinary course and have not entered into any material oral or written agreement or other material transaction that is not in the ordinary course of business (other than this Agreement) or that could reasonably be expected to result in a Sen Lang Material Adverse Effect; (ii) no Acquired Company has sustained any material loss or interference with its business or properties from fire, flood, windstorm, accident, strike or other calamity (whether or not covered by insurance); (iii) there has been no material change in the indebtedness of the Acquired Companies, no change in the capital stock of Sen Lang and no dividend or distribution of any kind declared, paid or made by Sen Lang on any class of its capital stock; (iv) there has been no event or condition which has caused a Sen Lang Material Adverse Effect, nor any development, occurrence or state of facts or circumstances known to Sen Lang that could, singly or in the aggregate, reasonably be expected to result in a Sen Lang Material Adverse Effect; and (v) there has been no material change by any Acquired Company in its accounting principles, practices or methods.
3.12.2 Since December 31, 2018, other than in the ordinary course of business consistent with past practice, there has not been any increase in the compensation or other benefits payable, or which could become payable, by Sen Lang, to its officers or key employees, or any amendment of any of the Sen Lang Employee Benefit Plans.
A-9
3.13 Capitalization.
3.13.1 The authorized share capital of Sen Lang consists solely of 50,000 Sen Lang Shares, par value $1.00 per Share. As of the date hereof there are 10,001 Sen Lang Shares outstanding and no Sen Lang Shares are held in Sen Lang’s treasury. There are no warrants or options outstanding, and no Sen Lang Shares are reserved for issuance for any purpose. There are not any existing options, warrants, calls, subscriptions, or other rights or other agreements or commitments obligating Sen Lang to issue, transfer or sell any Sen Lang Shares or any other securities convertible into or evidencing the right to subscribe for any such Sen Lang Shares. There are no outstanding stock appreciation rights with respect to the Sen Lang Shares. All issued and outstanding Sen Lang Shares are duly authorized and validly issued, fully paid and nonassessable and have not been issued in violation of (nor are any of the Sen Lang Shares, or other equity interests in, Sen Lang subject to) any preemptive or similar rights created by statute, the certificate of incorporation or by-laws of Sen Lang or any agreement to which Sen Lang is a party or by which it may be bound.
3.13.2 Except as set forth in Section 3.13.2 of the Sen Lang Disclosure Schedule, there are no (i) obligations, contingent or otherwise, of the Acquired Companies to repurchase, redeem or otherwise acquire any Sen Lang Shares, or provide funds to, or make any investment in (in the form of a loan, capital contribution or otherwise), or provide any guarantee with respect to the obligations of, any other person, or (ii) agreements, arrangements or commitments of any character (contingent or otherwise) pursuant to which any person is or may be entitled to receive any payment based on the revenues or earnings (or any component thereof), or calculated in accordance therewith, of any Acquired Company. Section 3.13.2 of the Sen Lang Disclosure Schedule sets forth the contingent earn-out obligations to which any Acquired Company is subject. There are no voting trusts, proxies or other agreements or understandings to which any Acquired Company is a party or by which any Acquired Company is bound with respect to the voting of any shares of capital stock of any Acquired Company.
3.14 Capital Stock of Subsidiaries. The Subsidiaries of Sen Lang, each of which is wholly-owned by an Acquired Company or controlled by an Acquired Company by the Control Documents, are listed in Section 3.14 of the Sen Lang Disclosure Schedule. There are no proxies with respect to such shares, and there are not any existing options, warrants, calls, subscriptions, or other rights or other agreements or commitments obligating any Acquired Company to issue, transfer or sell any shares of capital stock of any Acquired Company or any other securities convertible into or evidencing the right to subscribe for any such shares. All of such shares so beneficially owned by Sen Lang are duly authorized and validly issued, fully paid, nonassessable and free of preemptive rights with respect thereto and are owned by Sen Lang, directly or indirectly, free and clear of any claim, lien or Encumbrance of any kind with respect thereto. Except as set forth in Section 3.14 of the Sen Lang Disclosure Schedule and the Control Documents, Sen Lang does not directly or indirectly own any interest in any corporation, partnership, limited liability company, joint venture or other business association or entity.
A-10
3.15 Litigation. Except as set forth in Section 3.15 of the Sen Lang Disclosure Schedule, as of the date hereof there are no material pending Actions or, to the knowledge of the Acquired Companies, investigations by, against or affecting any Acquired Company or any of their officers, directors, properties, assets or operations, or with respect to which any Acquired Company is responsible by way of indemnity or otherwise. Except as set forth in Section 3.15 of the Sen Lang Disclosure Schedule: (i) there are no material pending or, to the knowledge of the Acquired Companies, threatened Actions or investigations by, against or affecting any Acquired Company or any of their officers, directors, properties, assets or operations, or with respect to which they are responsible by way of indemnity or otherwise; and (ii) to the knowledge of the Acquired Companies, there are no material Actions or investigations are threatened or contemplated and there is no reasonable basis, to the knowledge of Sen Lang, for any such Actions or investigation, whether or not threatened or contemplated.
3.16 Insurance. Sen Lang has insurance policies and fidelity bonds covering each Acquired Company’s assets, business, equipment, properties, operations, employees, officers and directors which Sen Lang reasonably and in good faith believes are adequate to conduct the business of the Acquired Companies. All premiums due and payable under all such policies and bonds have been paid, and Sen Lang is otherwise in full compliance with the terms and conditions of all such policies and bonds, except where the failure to have made payment or to be in full compliance would not, individually or in the aggregate with all such other failures, have a Sen Lang Material Adverse Effect. Sen Lang reasonably believes that the reserves established by the Acquired Companies in respect of all matters as to which any Acquired Company self-insures or carries retention and/or deductibles, including workers’ medical coverage and workers’ compensation, are adequate and appropriate, and no Acquired Company is aware of any facts or circumstances existing as of the date hereof that would reasonably be expected to cause such reserves to be materially inadequate or inappropriate.
3.17 Title to and Condition of Properties. The Acquired Companies have good title to all of the real property and personal property reflected on the March 31, 2021 unaudited consolidated balance sheet of the Acquired Companies (the “Sen Lang Balance Sheet”), except for property since sold or otherwise disposed of in the ordinary course of business and consistent with past practice and except for defects of title which are not material to the Acquired Companies taken as a whole. Except as set forth on Section 3.17 of the Sen Lang Disclosure Schedule, no Acquired Company owns any real property. No real or tangible personal property owned or leased by any Acquired Company is subject to claims, liens or other encumbrances of any kind or character, including mortgages, pledges, liens, conditional sale agreements, charges, security interests, easements, restrictive covenants, rights of way or options, except for (i) Encumbrances for Taxes not yet delinquent or which are being contested in good faith by appropriate Action and in respect of which any Acquired Company has set aside on its books adequate reserves in accordance with generally accepted accounting principles; (ii) mechanics’, carriers’, workers’, repairers’, materialmen’s, landlords’ and other similar statutory or common law liens incurred in the ordinary course of business for obligations not yet delinquent or the validity of which is being contested in good faith by appropriate Action and in respect of which the appropriate Acquired Company has set aside on its books adequate reserves in accordance with generally accepted accounting principles; (iii) in the case of real property, easements, rights of way, restrictions, minor defects or irregularities in title that do not individually or in the aggregate have a material adverse effect on the value or use of the real property encumbered thereby as currently used in the operation of the business of the Acquired Companies; (iv) those which would not materially interfere with the conduct of the business of the Acquired Companies (the encumbrances described in clauses (i) through (iv) of this sentence, collectively, the “Sen Lang Permitted Encumbrances”); (v) those securing liabilities reflected in the Sen Lang Balance Sheet; or (vi) those described in Section 3.17(vi) of the Sen Lang Disclosure Schedule.
A-11
3.18 Leases. There have been delivered or made available to Avalon true and complete copies of each lease pursuant to which Real Property or personal property is held under lease by any Acquired Company (limited, in the case of personal property, to leases pursuant to which annual rentals are reasonably expected to be at least $100,000 per year), and true and complete copies of each lease pursuant to which an Acquired Company leases real or personal property to others (limited in the case of personal property, to leases pursuant to which annual rentals are reasonably expected to be at least $100,000 per year). Section 3.18 of the Sen Lang Disclosure Schedule sets forth a true and complete list of all such leases, and such leases are the only leases that are material to the business conducted by the Acquired Companies taken as a whole. All of the leases so listed (i) are, in all material respects, valid and subsisting and in full force and effect with respect to the Acquired Companies, as the case may be, and, to Sen Lang’s knowledge, with respect to any other party thereto and (ii) were entered into as a result of bona fide arm’s length negotiations with the other party or parties thereto. The Acquired Companies have valid leasehold interests in all properties leased thereunder free and clear of all material liens and encumbrances other than Sen Lang Permitted Encumbrances. The real properties leased by the Acquired Companies are, in all material respects, in good operating order and condition, subject to ordinary wear and tear. To the knowledge of The Acquired Companies, there are no material structural, mechanical or other defects in any improvements located on such real properties.
3.19 Contracts and Commitments. Except as set forth in Section 3.19 of the Sen Lang Disclosure Schedule, no Acquired Company is a party to any existing contract, obligation or commitment of any type in any of the following categories:
3.19.1 contracts for the purchase by the Acquired Companies of medicines, materials, supplies or equipment which are not cancelable upon 90 days’ or less notice and which either (i) have not been entered into in the ordinary course of business and consistent with past practice or (ii) provide for purchase prices substantially greater than those presently prevailing for such materials, supplies or equipment, or (iii) contracts obligating the Acquired Companies to make capital expenditures in excess of $50,000;
3.19.2 contracts under which the Acquired Companies has, except by way of endorsement of negotiable instruments for collection in the ordinary course of business and consistent with past practice, become absolutely or contingently or otherwise liable for (i) the performance of any other person, firm or corporation under a contract, or (ii) the whole or any part of the indebtedness or liabilities of any other person, firm or corporation;
3.19.3 powers of attorney outstanding from the Acquired Companies other than as issued in the ordinary course of business and consistent with past practice with respect to customs, insurance, patent, trademark or tax matters, or to agents for service of process;
A-12
3.19.4 contracts under which any amount payable by the Acquired Companies is dependent upon, or calculated in accordance with, the revenues or earnings (or any component thereof) of any Acquired Company;
3.19.5 contracts with any director, officer, employee or Affiliate (as defined herein) of the Acquired Companies other than in such person’s capacity as a director, officer or employee of an Acquired Company;
3.19.6 contracts which limit or restrict where any Acquired Company may conduct its business or the type or line of business in which any Acquired Company may engage;
3.19.7 contracts with any party for the loan of money or availability of credit to or from any Acquired Company (except credit extended by an Acquired Company to customers in the ordinary course of business and consistent with past practice);
3.19.8 any material hedging, option, derivative or other similar transaction; or
3.19.9 contracts pursuant to which any of the Acquired Companies (a) grants any Person any exclusive license, exclusive option or other exclusive right with respect to any Sen Lang Intellectual Property, (b) grants any Person any license, option or other right with respect to any Sen Lang Intellectual Property that is material to the business of the Acquired Companies or (c) is granted any license, option or other right with respect to any Sen Lang Intellectual Property that is material to the business of the Acquired Companies.
True and complete copies of all contracts, obligations and commitments listed in Section 3.19 of the Sen Lang Disclosure Schedule have been delivered or made available to Avalon. None of the Acquired Companies or, to the knowledge of the Acquired Companies, any other party is in breach of or default under any of the contracts, obligations and commitments listed in Section 3.19 of the Sen Lang Disclosure Schedule or under any other Sen Lang Contracts (and, to the knowledge of Sen Lang, no facts or circumstances exist which could reasonably support the assertion of any such breach or default) except for breaches and defaults which would not, singly or in the aggregate with all other such breaches, have a Sen Lang Material Adverse Effect.
3.20 Employees; Labor Matters. Except as set forth in Section 3.20 of the Sen Lang Disclosure Schedule, no Acquired Company is a party to or bound by any collective bargaining agreement, and there are no labor unions or other organizations representing, purporting to represent or attempting to represent any employees employed by the Acquired Companies thereof. Since January 1, 2018, there has not occurred or been threatened any material strike, slowdown, picketing, work stoppage, concerted refusal to work overtime or other similar labor activity with respect to any employees of the Acquired Companies thereof. Except as set forth in Section 3.20 of the Sen Lang Disclosure Schedule, there are no labor disputes currently subject to any grievance procedure, arbitration or litigation and there is no representation petition pending or threatened with respect to any employee of any Acquired Company. Each of the Acquired Companies has complied with Applicable Law pertaining to the employment or termination of employment of their respective employees, including all such Applicable Laws relating to labor relations, equal employment opportunities, fair employment practices, prohibited discrimination or distinction and other similar employment activities. Contributions required to be made by employers under Applicable Law to all the mandatory social welfare and pension funds in respect of all employees of the Acquired Companies have been duly and punctually paid in full. The Acquired Companies have signed labor contracts and confidential and non-compete agreements (containing proprietary information protection and invention-creation ownership clauses) with all of their employees in forms and content in compliance with the Applicable Laws.
A-13
3.21 No Change of Control Puts. Except as described in Section 3.21 of the Sen Lang Disclosure Schedule, neither the execution and delivery by Sen Lang of this Agreement nor the consummation of the Acquisition or any other transaction contemplated hereby gives rise to any obligation of any Acquired Company to, or any right of any holder of any security of an Acquired Company to require any Acquired Company to, purchase, offer to purchase, redeem or otherwise prepay or repay any such security, or deposit any funds to effect the same.
3.22 Employment and Labor Contracts. Except as set forth in Section 3.22 of the Sen Lang Disclosure Schedule, neither the Acquired Companies is a party to any employment, management services, consultation or other contract or agreement with any past or present officer, director or employee or, to the knowledge of Sen Lang, any entity affiliated with any past or present officer, director or employee, other than the agreements executed by employees generally, the forms of which have been provided to Avalon.
3.23 Intellectual Property Rights.
3.23.1 The Acquired Companies own or have the right to use all Sen Lang Intellectual Property Rights (as defined herein) necessary to the conduct of their respective businesses. Subject to obtaining any associated consents with respect to agreements or licenses listed in Section 3.3.2 of the Sen Lang Disclosure Schedule, each Sen Lang Intellectual Property Right owned or used by the Acquired Companies immediately prior to the Closing will be owned or available for use by the Surviving Company or its subsidiaries on substantially the same terms and conditions immediately subsequent to the Closing. Section 3.23 of the Sen Lang Disclosure Schedule contains a list of all patents, trade names, registered copyrights, trademarks and service marks, mask works and applications for the foregoing owned by the Acquired Companies. Except as set forth in Section 3.23 of the Sen Lang Disclosure Schedule, (i) the Acquired Companies have sole and exclusive ownership of, and valid and unencumbered (except for Sen Lang Permitted Encumbrances) title to, the Sen Lang Intellectual Property Rights set forth in such Section 3.23 and, to the knowledge of the Acquired Companies, such title has not been challenged (pending or threatened) by others except for the encumbrances listed therein; (ii) there have been no claims or assertions made by others that any Acquired Company has infringed or misappropriated any Intellectual Property Rights of others by the development, manufacture or sale of products, the rendering of services or any other activity since December 31, 2016; (iii) to the knowledge of Sen Lang, there has been no such infringement or misappropriation by any Acquired Company since December 31, 2016; (iv) the Acquired Companies have no knowledge of any infringement or misappropriation of Sen Lang Intellectual Property Rights of any Acquired Company by others; and (v) all Sen Lang Intellectual Property Rights owned by the Acquired Companies (a) are in good standing with the registration authority therefore, if any, (b) to the extent recorded on the public record, are recorded in the name of the Acquired Companies and (c) have been duly registered with, filed in or issued by, as the case may be, the State Intellectual Property Office of the PRC, General Administration of Press and Publication of the PRC and Trademark Bureau of Stated Administration For Industry & Commerce and other filing offices in the PRC, and the U.S. Patent and Trademark Office and the U.S. Copyright Office and other filing offices, domestic or foreign, to the extent necessary or desirable to ensure full protection under Applicable Law, and the same remain in full force and effect. True and complete copies of all material listed in Section 3.23 of the Sen Lang Disclosure Schedule have been delivered or made available to Avalon.
A-14
3.23.2 Notwithstanding the above, the Sen Lang Intellectual Property Right owned or used by the Acquired Companies immediately prior to the Closing does not incorporate subject matter obtained from other entities or individuals (including but not limited to Dr. David Maloney, Shenzhen Geno-Immune Medical Institute, Fred Hutchinson Cancer Research Center, City of Hope Cancer Research Center) that would result in the entity or individual having an ownership right to the Sen Lang Intellectual Property Right.
3.23.3 Sen Lang does not presently have or did not have in the past any relationship with the Chinese People’s Liberation Army (PLA) or other military controlled entity, including but not limited to receipt of funding, sharing of research, conducting trials and research or by an employee or director (including Sun Zhiqiang) being active in the PLA while associated with Sen Lang (other than Sun Zhiqiang’s association with PLA 302 Hospital) ; and the PLA, PLA 302 Hospital or other military entity does not have an ownership right to the Sen Lang Intellectual Property Right.
3.23.4 Sen Lang does not presently have or did not have in the past any relationship with the Chinese Government (including local governments such as Shijiazhung Municipal Government) that would result in the Chinese Government having an ownership right to the Sen Lang Intellectual Property Right.
3.23.5 Sen Lang does not presently have or did not have in the past any relationship with a Chinese government-funded research institute that would result in the institute having an ownership right to the Sen Lang Intellectual Property Right.
3.23.6 All shareholder names (including individuals and entities) and percent ownership of Sen Lang have been disclosed to Avalon. There are not out-license to or in-licenses from any inventor and no investor has an ownership right to the Sen Lang Intellectual Property Right.
3.23.7 Sen Lang, including any employee or director did not received any U.S funding in violation of National Institues of Health (NIH) regulations.
3.23.8 Sen Lang has not licensed any technology to Sinopharm Group and does not have any arrangement with Sinopharm Group that impacts Sen Lang Intellectual Property Right.
3.24 Taxes.
3.24.1 All Returns required to be filed by, or with respect to any activities or assets of, each of the Acquired Companies have been duly and timely filed, and are correct and complete in all material respects and have been prepared in accordance with Applicable Laws. All Taxes due and owning by the Acquired Companies (whether or not shown as owing on any Return) have been paid. No Acquired Company is currently the beneficiary of any extension of time within which to file any Return.
A-15
3.24.2 Each of the Acquired Companies has duly and timely withheld and paid all Taxes required to be withheld and paid in connection with any amounts paid or owing to any employee, independent contractor, creditor, stockholder, or other third party. Each of the Acquired Companies has properly collected and remitted all sales, use, value added, and similar Taxes with respect to sales or leases made to, purchases made from, or services provided to its customers or have properly received and retained any appropriate Tax exemption certificates and other documentation for all sales, leases, or purchases made, or services provided, without charging or remitting sales, use, value added, or similar Taxes that qualify such sales, leases, purchases, or services as exempt from sales, use, value added, and similar Taxes.
3.24.3 No claim has ever been made by an authority in a jurisdiction where an Acquired Company does not file Returns that such Acquired Company is or may be subject to taxation by that jurisdiction. There are no liens for Taxes (other than Taxes not yet due and payable) upon any of the assets of the Acquired Companies. No Tax audits or administrative or judicial Tax proceedings are pending or being conducted with respect to the Acquired Companies. None of the Acquired Companies has received from any taxing authority (including jurisdictions where the Acquired Companies have not filed Returns) any (i) notice indicating an intent to open an audit or other review, (ii) request for information related to Tax matters, or (iii) notice of deficiency or proposed adjustment for any amount of Tax proposed, asserted, or assessed by any taxing authority against any Acquired Company.
3.24.4 Section 3.24.4 of the Sen Lang Disclosure Schedule lists all Income Tax Returns (as defined herein) that have been filed with respect to OpCo (for taxable periods beginning on 2019) and Sen Lang Lab (for taxable periods beginning on 2020), indicates those Returns that have been audited, and indicates those Returns that currently are the subject of audit.
3.24.5 No Acquired Company has (i) waived any statute of limitations in respect of Taxes, (ii) agreed to any extension of time with respect to a Tax assessment, deficiency or collection or (iii) executed or filed any power of attorney with respect to Taxes, which waiver, agreement or power of attorney is currently in force.
3.24.6 Except as set forth in Section 3.24.6 of the Sen Lang Disclosure Schedule, (i) there are no outstanding adjustments for Tax purposes applicable to any Acquired Company required as a result of changes in methods of accounting effected on or before the date of this Agreement and (ii) no material Tax elections have been made by any Acquired Company that are currently in force or by which any Acquired Company is bound.
3.24.7 Except as set forth in Section 3.24.7 of the Sen Lang Disclosure Schedule, no Acquired Company (i) is a party to or bound by or has any obligation under any Tax allocation, sharing, indemnity or similar agreement or arrangement or (ii) is or has been a member of any group of companies filing a consolidated, combined or unitary Income Tax Return.
A-16
3.24.8 The unpaid Taxes of the Acquired Companies did not, as of the date of the Sen Lang Balance Sheet, exceed the reserve for Tax liability (rather than any reserve for deferred Taxes established solely to reflect timing differences between book and Tax income) set forth on the Sen Lang Balance Sheet, and the unpaid Taxes of the Acquired Companies will not, as of the Closing Date, exceed that reserve as adjusted for the passage of time through the Closing Date in accordance with the past custom and practice of the Acquired Companies in filing their Returns. Since the date of the Sen Lang Balance Sheet, no Acquired Company has incurred any liability for Taxes arising from extraordinary gains or losses, as such term is used in thee International Financial Reporting Standards (“IFRS”), except in the ordinary course of business.
3.24.9 None of the Acquired Companies will be required to include any item of income in, or exclude any item of deduction from, taxable income for any taxable period (or portion thereof) ending after the Closing Date as a result of: (i) any change in method of accounting for a taxable period ending on or prior to the Closing Date, (ii) any “closing agreement” as described in Section 7121 of the Code (or any corresponding or similar provision of state, local or non-U.S. Tax Law) executed on or prior to the Closing Date, (iii) any installment sale or open transaction disposition made on or prior to the Closing Date, (iv) any intercompany transaction or excess loss account described in Treasury Regulations under Section 1502 of the Code (or any corresponding or similar provision of state, local, or non-U.S. Tax Law), (v) any use of an improper method of accounting for a taxable period ending on or prior to the Closing Date, (vi) any prepaid amounts received or deferred revenue accrued on or prior to the Closing Date, (vii) any related party transactions subject to any transfer pricing arrangements involving any Acquired Company entered into on or prior to the Closing Date, (viii) any loan pursuant to Section 1102 of the CARES Act or similar U.S. or non-U.S. governmental program, or (ix) an election under Section 108(i) of the Code (or any similar provision of state, local or non-U.S. Law).
3.24.10 The Acquired Companies are in compliance in all material respects with all applicable transfer pricing laws and regulations, including the execution and maintenance of contemporaneous documentation substantiating the transfer pricing practices and methodology and conducting intercompany transactions at arm’s length. All transactions entered into by the Acquired Companies are not and will not become subject to an adjustment in accordance with any transfer pricing rules in any relevant jurisdiction.
3.24.11 None of the Acquired Companies is currently nor has ever previously been classified as (i) a “passive foreign investment company” within the meaning of Section 1297 of the Code or (ii) a “controlled foreign corporation” within the meaning of Section 957 of the Code. None of the Acquired Companies has a permanent establishment or otherwise has an office, branch or fixed place of business in a country other than the country in which it is organized and is and has at all times been exclusively resident for all Tax purposes and subject to Tax in its jurisdiction of formation only. None of the Acquired Companies has requested or is the subject of or bound by any private letter ruling, technical advice memorandum, or similar ruling or memorandum with any taxing authority with respect to any material Taxes, nor is any such request outstanding.
A-17
3.25 Employee Benefit Plans
3.25.1 Except as set forth in Section 3.25 of the Sen Lang Disclosure Schedule, with respect to any employee or former employee of any Acquired Company, none of the Acquired Companies, or any Affiliated company presently maintains, contributes to or has any liability under: (i) any bonus, incentive compensation, profit sharing, retirement, pension, group insurance, death benefit, cafeteria, medical expense reimbursement, dependent care, stock option, stock purchase, stock appreciation rights, deferred compensation, consulting, severance pay or termination pay, vacation pay, welfare or other employee benefit or fringe benefit plan, program or arrangement; or (ii) any plan, program or arrangement which is an employee pension benefit plan, or an “employee welfare benefit plan” as defined under relevant laws, including laws of the PRC applicable to any Acquired Company. Each plan, program and arrangement set forth in Section 3.25 of the Sen Lang Disclosure Schedule is herein referred to as a “Sen Lang Employee Benefit Plan.” The term “affiliated company” means any organization that would be aggregated with any Acquired Company under Section 414(b), (c), (m) or (o) of the Code.
3.25.2 There is no pending or threatened Action or investigation against or involving any Sen Lang Employee Benefit Plan (other than routine claims for benefits) and there is no basis for any facts which could give rise to any such Action or investigation.
3.25.3 None of the Acquired Companies nor any of their affiliates is a party to any employment agreement, whether written or oral, or agreement with change in control or similar provisions, or a collective bargaining agreement or contract with any labor union relating to any employees or former employees of any Acquired Company.
3.26 Environmental Matters.
3.26.1 Each of the Acquired Companies has complied and is in compliance in all material respects with all applicable Environmental Laws pertaining to any of the properties and assets of the Acquired Companies (including all real property owned by any Acquired Company, together with all structures, facilities, improvements, fixtures, systems, equipment and items of property presently or hereafter located thereon or attached or appurtenant thereto or owned by any Acquired Company and located on real property leased by any Acquired Company, and all easements, licenses, rights and appurtenances relating to the foregoing (collectively, the “Sen Lang Real Property”) and the use and ownership thereof, and to the operation of their respective businesses. No material violation by any Acquired Company is being alleged of any applicable Environmental Law relating to any of the properties and assets of any Acquired Company (including the Sen Lang Real Property) or the use or ownership thereof, or to the operation of their respective businesses.
3.26.2 No Acquired Company or any other Person (including any tenant or subtenant) has caused or taken any action that will result in, nor is any Acquired Company subject to, any material liability or obligation on the part of any Acquired Company or any Affiliates of any Acquired Company, relating to (x) the environmental conditions on, under, or about the Sen Lang Real Property or other properties or assets owned, leased, operated or used by any Acquired Company or any predecessor thereto at the present time or in the past, including the air, soil and groundwater conditions at such properties or (y) the past or present use, management, handling, transport, treatment, generation, storage, disposal or Release of any Hazardous Materials.
A-18
3.26.3 Sen Lang has disclosed and made available to Avalon all information, including all studies, analyses and test results, in the possession, custody or control of or otherwise known to any Acquired Company relating to (x) the environmental conditions on, under or about the Real Property or other properties or assets owned, leased, operated or used by any Acquired Company or any predecessor in interest thereto at the present time or in the past, and (y) any Hazardous Materials used, managed, handled, transported, treated, generated, stored or Released by any Acquired Company or any other Person on, under, about or from any of the Sen Lang Real Property, or otherwise in connection with the use or operation of any of the properties and assets of any Acquired Company or their respective businesses.
3.27 [INTENTIONALLY OMITTED.]
3.28 Security Holders’ Identity and Compliance. Each holder of any equity interest in the OpCo is a citizen and permanent resident of the PRC and did not hold and does not hold any identification that may require the registration of the OpCo as a foreign invested enterprise pursuant to Applicable Laws of the PRC in effect at and from the time of the incorporation of the OpCo through the date of the Closing. The Sen Lang Beneficial Shareholders and the Sen Lang Shareholders have complied with all Applicable Law (including Circular 37) and completed all procedures and requirements with respect to all of the Sen Lang’s financing transactions. Neither the Acquired Companies nor any of the Sen Lang Parties has received any oral or written inquiries, notifications, orders or any other forms of official correspondence from SAFE with respect to any actual or alleged non-compliance with Circular 37 and each PRC Company has made all oral or written filings, registrations, reporting or any other communications required by SAFE or any of its local branches. “Circular 37” means the Notice of the State Administration of Foreign Exchange on the Administration of Foreign Exchange Involved in Overseas Investment, Financing and Round-trip Investment Conducted by Residents in China via Special-Purpose Companies (关于境内居民通过特殊目的公司境外投融资及返程投资外汇管理有关问题的通知). “SAFE” means PRC State Administration of Foreign Exchange or any of its local branches.
3.29 Other Representations and Warranties Relating to the PRC Companies.
3.29.1 The constitutional documents and certificates and related contracts and agreements of each of the PRC Companies are valid and have been duly approved or issued (as applicable) by competent PRC authorities.
3.29.2 All approvals required under PRC law for the due and proper establishment and operation of each of the PRC Companies have been duly obtained from the relevant PRC authorities and are in full force and effect.
3.29.3 All filings and registrations with the PRC authorities required in respect of each of the PRC Companies and its operations have been duly completed in accordance with the relevant rules and regulations.
3.29.4 Except as set forth in Section 3.29.4 of the Sen Lang Disclosure Schedule, the registered capitals of the PRC Companies are fully paid up. The HK Subsidiary legally and beneficially owns 100% of the equity interest in the PRC Subsidiary. There are no outstanding rights, or commitments made by any of the PRC Companies to sell any of its equity interest, except as provided in the Control Documents.
A-19
3.29.5 All of the equity interests in OpCo are legally, beneficially and ultimately owned by the Sen Lang Beneficial Shareholders. None of the Sen Lang Beneficial Shareholders is, in any manner or through any arrangement, holding any equity interest in OpCo on behalf of or for the benefit of any third party. Except as provided in the Control Documents, there is no agreement for concerted action, entrustment agreement or other similar arrangements among the Sen Lang Beneficial Shareholders.
3.29.6 Neither of the PRC Companies is in receipt of any letter or notice from any relevant authority notifying revocation of any permits or licenses issued to it for non-compliance or the need for compliance or remedial actions in respect of the activities carried out directly or indirectly by it.
3.29.7 Each of the PRC Companies has been conducting and will conduct its business activities within the permitted scope of business or is otherwise operating its business in full compliance with all relevant legal requirements and with all requisite approvals granted by competent PRC authorities.
3.29.8 In respect of approvals requisite for the conduct of any part of the business of each of the PRC Companies which are subject to periodic renewal, there is no reason to believe that such requisite renewals will not be timely granted by the relevant PRC authorities.
3.29.9 With regard to employment and staff or labor management, each of the PRC Companies has complied with all applicable PRC laws and regulations in all material respects, including without limitation, laws and regulations pertaining to welfare funds, social benefits, medical benefits, insurance, retirement benefits, and pensions.
3.29.10 Each of the Control Documents has been duly executed and delivered by the parties thereto and has remained effective, legal, valid and enforceable in accordance with the terms and conditions therein. Pursuant to the Control Documents, the OpCo has been effectively controlled by Sen Lang through the PRC Subsidiary and the HK Subsidiary, 100% of the economic benefits of the operations of the OpCo have been and will be received by Sen Lang, and the financial statements of the OpCo can be consolidated into the financial statements of Sen Lang as a special purpose vehicle under IFRS, together with its pronouncements thereon from time to time, and applied on a consistent basis. No oral or written inquiries, notifications or any other form of official correspondence has been issued by any government authorities challenging or questioning the legality or enforceability of any of the Control Documents.
3.29.11 The PRC Companies have made full contributions to the social insurance fund and the provident fund for all of their employees.
3.30 Captive Structure. The Control Documents, upon execution, will constitute valid and binding obligations of the parties thereto and are adequate to establish and maintain the intended captive structure, under which the financial statements of the OpCo can be consolidated with those of the other Acquired Companies in accordance with the then duly adopted accounting principles of Sen Lang. No oral or written inquiries, notifications or any other form of official correspondence has been issued by any government authorities challenging or questioning the legality or enforceability of any of the Control Documents.
A-20
3.31 Foreign Government Ownership Under CFIUS. No Acquired Company is owned or controlled, directly or indirectly, by any foreign government such that, as a result of the transaction contemplated in this Agreement, a foreign government would acquire a “substantial interest,” as defined in 31 C.F.R. Part 800.244, in a U.S. business.
3.32 No Sanctioned Persons. The Sen Lang Owners represent that no Sen Lang Party is a Sanctioned Person, senior foreign political figure, person entrusted with prominent public function in a foreign country, or person convicted of or charged with any criminal act.
3.33 Disclosure. All information disclosed by or on behalf of any Acquired Company to Avalon or its advisers on or prior to the date hereof is true and accurate in all material aspects, and the Acquired Companies are not aware of any other fact or matter which renders any such information misleading because of any omission, ambiguity or for any other reason. All information contained in the Disclosure Schedule is true and accurate in all aspects and fairly presented and there is no fact or matter which has not been disclosed in the Sen Lang Disclosure Schedule which renders any such information untrue or misleading and there is no fact or matter concerning any Acquired Company which has not on the basis of the utmost good faith been disclosed in the Sen Lang Disclosure Schedule which would reasonably be expected to influence the decision of Avalon to proceed with the Acquisition on the terms and conditions thereof.
ARTICLE IV.
REPRESENTATIONS AND WARRANTIES OF AVALON
Except as set forth in the Disclosure Schedule delivered by Avalon to the Sen Lang Representative at or prior to the execution of this Agreement (the “Avalon Disclosure Schedule”) (each section of which qualifies the correspondingly numbered representation and warranty, regardless of whether such representation or warranty expressly refers to or is qualified by reference to such Avalon Disclosure Schedule) or the Avalon SEC Reports, Avalon represents and warrants to the Sen Lang Shareholders as follows:
4.1 Organization and Qualification.
4.1.1 Each of Avalon and its Subsidiaries (as defined in Section 4.1.2) is an entity duly incorporated, validly existing and in good standing under the laws of the jurisdiction of its incorporation and has the corporate power and authority to own, lease and operate its properties and to conduct its business as described in the Avalon SEC Reports (as defined herein). Each of Avalon and each of Avalon’s Subsidiaries is duly qualified to transact business as a foreign corporation or other foreign entity and is in good standing in each jurisdiction in which the conduct of its business or the ownership, leasing or operation of its property requires such qualification, except for failures to be so qualified or in good standing which would not, singly or in the aggregate with all such other failures, have an Avalon Material Adverse Effect. “Avalon Material Adverse Effect” means, with respect to any event, occurrence, matter, failure of event or occurrence, change, effect, state of affairs, breach, default, violation, fine, penalty or failure to comply (each, a “Circumstance”), individually or taken together with all other Circumstances contemplated by or in connection with any or all of the representations and warranties made in this Agreement, a material adverse effect on the business, assets (including intangible assets), liabilities (contingent or otherwise), financial condition, results of operations or prospects of Avalon and its Subsidiaries, taken as a whole; provided, however, that Avalon Material Adverse Effect shall not be deemed to include the impact of: (A) the implementation of changes in generally accepted accounting principles; (B) actions and omissions of Avalon or its Subsidiaries taken or permitted with the prior written consent of Sen Lang after the date hereof; (C) expenses reasonably incurred by Avalon or its Subsidiaries in consummating the transactions contemplated by this Agreement; (D) changes in the general economic or financial market conditions; (E) any occurrence, condition, change, event or effect that affects the biotechnology industry generally; and (F) acts of God, war, acts of terrorism, epidemics, pandemics, quarantines, injunctions or restraining orders, failure of any public authority or governmental body or agency to issue any permit or license, or generalized lack of raw materials or energy.
A-21
4.1.2 Neither Avalon nor any of its Subsidiaries is in violation of any of the provisions of its certificate of incorporation or by-laws, or other similar organizational documents, each as amended and currently in effect, or, if it is a limited liability company or partnership, its operating agreement, partnership agreement or other comparable agreement. True and complete copies of the certificate of incorporation and by-laws, each as amended and as currently in effect, of Avalon and true and complete copies of the certificate of incorporation and by-laws, or other similar organizational documents, each as amended and currently in effect, of each Subsidiary of Avalon have been previously delivered or made available to Sen Lang.
4.2 Authority Relative to this Agreement. Avalon has the corporate power and authority to execute and deliver this Agreement and, upon obtaining the approval of a majority of the outstanding shares of Avalon Common Stock at the Avalon Annual Meeting or any adjournment thereof as authorized under the DGCL, to consummate the Acquisition and the other transactions contemplated hereby. The execution and delivery of this Agreement and the consummation of the Acquisition and the other transactions contemplated hereby have been duly and validly authorized by the Boards of Directors of Avalon, and except as stated in the preceding sentence, no other corporate proceedings on the part of Avalon are necessary to authorize this Agreement or to consummate the Acquisition and the other transactions contemplated hereby. This Agreement has been duly and validly executed and delivered by Avalon and, assuming the due authorization, execution and delivery hereof by Sen Lang, the Sen Lang Shareholders, the Sen Lang Beneficial Shareholders, and the Sen Lang Represetnative, and subject to shareholder approval of Sen Lang, constitutes a valid and binding agreement of Avalon, enforceable against Avalon in accordance with its terms, except to the extent that its enforceability may be limited by applicable bankruptcy, insolvency, reorganization, moratorium or other laws affecting the enforcement of creditors’ rights generally or by general equitable principles.
4.3 Consents, No Conflicts.
4.3.1 Except for actions to be taken in connection with (a) filings required pursuant to any state securities or “blue sky” laws, (b) filings and other matters relating to the listing on Nasdaq of the shares of Avalon Common Stock required to be issued pursuant to this Agreement, (c) notices to or filings with the IRS or the Pension Benefit Guaranty Corporation (“PBGC”) with respect to any employee benefit plans (to the extent such notices to and filings with the IRS or the PBGC are described in Section 4.3.1 of the Avalon Disclosure Schedule) and (d) any other filings, notices, disclosures or registrations set forth in Section 4.3.1 of the Avalon Disclosure Schedule, no filing or registration with, notification or disclosure to, or permit, authorization, consent or approval of, (x) any court, (y) any government agency or body or (z) any third party, whether acting in an individual, fiduciary or other capacity, is required for the consummation by Avalon of the Acquisition or the other transactions contemplated hereby.
A-22
4.3.2 Except as set forth in Section 4.3.2 of the Avalon Disclosure Schedule, the execution, delivery and performance of this Agreement and the consummation of the Acquisition and the other transactions contemplated hereby and compliance by Avalon with any of the provisions hereof do not and will not: (i) subject to obtaining the approval of the Acquisition by holders of the Avalon Common Stock and the Avalon Preferred Stock, conflict with or result in any breach or violation of any provision of the certificate of incorporation or by-laws, or other similar organizational documents, each as amended, of Avalon or any of its Subsidiaries or (ii) result in (1) a breach or violation of, a default under or an event triggering any payment, obligation or acceleration of any obligation pursuant to any Avalon Employee Benefit Plan (as defined herein) or any grant or award made under any of the foregoing, (2) a breach or violation of, a default under or an event triggering a right of termination of, a default under, or the acceleration of any obligation or the creation of a lien, pledge, security interest or other encumbrance on assets (with or without the giving of notice or the lapse of time or both) pursuant to any provision of, any agreement, lease of real or personal property, marketing agreement, contract, note, mortgage, indenture or other obligation of Avalon or any of its Subsidiaries (“Avalon Contracts”) or, subject to making all filings, notifications and disclosures and receipt of all permits, authorizations, consents and approvals referred to in clauses “a” through “d” of Section 4.3.1 or in Section 4.3.1 of the Avalon Disclosure Schedule, any law, rule, ordinance or regulation or judgment, decree, order or award to which Avalon or any of its Subsidiaries is subject or any governmental or non-governmental authorization, consent, approval, registration, franchise, license or permit under which Avalon or any of its Subsidiaries conducts any of its business, or (3) any other change in the rights or obligations of any party under any of the Avalon Contracts, except, with respect to this clause (ii), for breaches, violations, defaults, triggering events, creations of liens, pledges, security interests or other encumbrances on assets, or changes in rights or obligations which would not, singly or in the aggregate with all other such matters, have an Avalon Material Adverse Effect.
4.3.3 As of the date of execution of this Agreement, Avalon has not received any de-listing notice from Nasdaq with respect to the Avalon Common Stock.
4.4 Board Recommendation. The Board of Directors or an appropriate committee of the Board of Directors of Avalon has, by unanimous written consent, approved and adopted this Agreement, the Acquisition and the other transactions contemplated hereby. At such meeting, the Board of Directors of Avalon or board committee determined that the terms of the Acquisition are fair to the holders of Avalon Common Stock and recommended that the holders of such shares approve and adopt this Agreement, the Acquisition, the issuance of the Avalon Common Stock pursuant to this Agreement and the other transactions contemplated hereby (the “Avalon Board Recommendation”).
4.5 Stockholder Protection Rights Agreements. Other than the Confidentiality Agreement (as defined herein), there are no contracts between Avalon, on the one hand, and any member of Sen Lang’s management or directors, on the other hand, as of the date hereof that relate in any way to Sen Lang or the transactions contemplated by this Agreement. Prior to the Board of Directors of Sen Lang approving this Agreement, the Acquisition and the other transactions contemplated hereby for purposes of the applicable provisions of the DGCL, Avalon, alone or together with any other person, was not at any time, or became, an “interested stockholder” thereunder or has taken any action that would cause the restrictions on business combinations with interested stockholders set forth in Section 203 of the DGCL to be applicable to this Agreement, the Acquisition, or any transactions contemplated by this Agreement. Avalon is not a party to any stockholder protection rights agreement or any agreement similar thereto.
A-23
4.6 No Existing Violation, Default, Etc. None of Avalon or its Subsidiaries is in violation of (A) Applicable Law or (B) any order, decree or judgment of any Governmental Authority having jurisdiction over Avalon or any of its Subsidiaries. No event of default or event that, but for the giving of notice or the lapse of time or both, would constitute an event of default, exists under any Avalon Contract or any lease, permit, license or other agreement or instrument to which Avalon or any of its Subsidiaries is a party or by which any of them is bound or to which any of the properties, assets or operations of Avalon or any of its Subsidiaries is subject.
4.7 Licenses and Permits. Each of Avalon and its Subsidiaries has such certificates, permits, licenses, franchises, consents, approvals, orders, authorizations and clearances from appropriate governmental agencies and bodies (“Avalon Licenses”) as are necessary to own, lease or operate its properties and to conduct its business in the manner described in the Avalon SEC Reports and as presently conducted and all such Avalon Licenses are valid and in full force and effect, other than any failure to have any such Avalon License or any failure of any such Avalon License to be valid and in full force and effect as would not, singly or in the aggregate with all such other failures, have an Avalon Material Adverse Effect. Avalon or one or more of its Subsidiaries are participants in the Medicaid program in the states listed in Section 4.7 of the Avalon Disclosure Schedule. Each of Avalon and its Subsidiaries is and, within the period of all applicable statutes of limitations, has been in compliance with its obligations under such Avalon Licenses and no event has occurred that allows, or after notice or lapse of time would allow, revocation or termination of such Avalon Licenses, other than any such failure to be in compliance with such obligations or any such revocation or termination as would not, singly or in the aggregate with all such other failures, revocations or terminations, have an Avalon Material Adverse Effect. Avalon has no knowledge of any facts or circumstances that could reasonably be expected to result in an inability of Avalon or any of its Subsidiaries to renew any material Avalon License. Subject to making all filings, notifications and disclosures and receipt of all permits, authorizations, consents and approvals referred to in Section 4.3.1 of the Avalon Disclosure Schedule, neither the execution and delivery by Avalon of this Agreement nor the consummation of any of the transactions contemplated herein will result in any revocation or termination of any material Avalon License.
4.8 Proxy Statement. None of the information supplied or to be supplied by Avalon for inclusion in, and none of the information regarding Avalon and its Subsidiaries incorporated by reference in, the Proxy Statement, including all amendments and supplements thereto, shall, on the date or dates the Proxy Statement is first mailed to stockholders of Avalon and the Sen Lang Shareholders and on the date or dates of the Avalon Annual Meeting and the Sen Lang Special Meeting, contain any untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary in order to make the statements therein, in the light of the circumstances under which they were made, not misleading. The Proxy Statement will comply as to form in all material respects with the applicable provisions of the Securities Act and the Exchange Act, as the case may be.
A-24
4.9 Finders or Brokers; Compensation Arrangements. Except as provided in Section 4.9 of the Avalon Disclosure Schedule, neither Avalon nor any Subsidiary of Avalon has employed any investment banker, broker, finder or intermediary in connection with the transactions contemplated hereby who might be entitled to a fee or any commission the receipt of which is conditioned in whole or part upon consummation of the Acquisition.
4.10 SEC Reports. Avalon has filed all forms, reports and documents required to be filed by it with the SEC since December 31, 2020 (the “Avalon Audit Date”) (including Avalon’s Annual Report on Form 10-K for the year ended December 31, 2020 and Avalon’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2021 and all certifications and statements required by Rule 13a-14 or 15d-14 under the Exchange Act or 18 U.S.C. §1350 (Section 906 of SOX) with respect to any Annual Reports or Proxy Statements), pursuant to the federal securities laws and the SEC’s rules and regulations thereunder, and SOX and all rules and regulations thereunder (collectively, and together with all forms, reports and documents filed by Avalon with the SEC after December 31, 2020, including any amendments thereto, the “Avalon SEC Reports”). Avalon SEC Reports were or will, as applicable, be prepared in accordance with the requirements of the Securities Act and the Exchange Act, as the case may be, and the rules and regulations thereunder. As of their respective dates, none of Avalon SEC Reports, including any financial statements or schedules included therein, contained or will contain, as applicable, any untrue statement of a material fact or omitted to state a material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they were or are made, as applicable, made, not misleading. No Subsidiary of Avalon is or has been required to file any form, report, registration statement or other document with the SEC.
4.11 Disclosure Controls and Procedures. Avalon maintains disclosure controls and procedures required by Rule 13a-15 or 15d-15 under the Exchange Act. Such controls and procedures are effective to ensure that all material information concerning Avalon and its Subsidiaries is made known on a timely basis to the individuals responsible for the preparation of Avalon’s filings with the SEC and other public disclosure documents. As used in this Section 4.11, the term “file” shall be broadly construed to include any manner in which a document or information is furnished, supplied otherwise made available to the SEC.
4.12 Financial Statements. The consolidated balance sheets and the related consolidated statements of income and cash flows (including the related notes thereto) of Avalon included in Avalon SEC Reports, as of their respective dates, complied in all material respects with applicable accounting requirements and the published rules and regulations of the SEC with respect thereto, were prepared in accordance with U.S. generally accepted accounting principles applied on a basis consistent with prior periods (except as otherwise noted therein), and present fairly in all material respects, the consolidated financial position of Avalon and its consolidated Subsidiaries as of their respective dates, and the consolidated results of their operations and their cash flows for the periods presented therein (subject, in the case of the unaudited interim financial statements, to notes and normal year-end adjustments that were not material in amount or effect).
A-25
4.13 SOX Certifications. The Chief Executive Officer and the Chief Financial Officer of Avalon have signed, and Avalon has furnished to the SEC, all certifications required by Sections 302 and 906 of SOX. Such certifications contain no qualifications or exceptions to the matters certified therein and have not been modified or withdrawn. Neither Avalon nor any of it officers has received notice from any Governmental Authority questioning or challenging the accuracy, completeness, form or manner of filing or submission of such certifications.
4.14 Undisclosed Liabilities. Except (i) as reflected in Avalon’s unaudited consolidated balance sheet at March 31, 2021 or liabilities described in any notes thereto, (ii) for liabilities incurred in the ordinary course of business since March 31, 2021 consistent with past practice or in connection with this Agreement or the transactions contemplated hereby, or (iii) performance obligations under contracts required in accordance with their terms, or performance obligations, to the extent required under Applicable Law, in each case to the extent arising after the date hereof, neither Avalon nor any of its Subsidiaries has any material liabilities or obligations of any nature (whether accrued, absolute, contingent or otherwise) and which, individually or in the aggregate, could reasonably be expected to have a Company Material Adverse Effect.
4.15 Off-Balance Sheet Arrangements. Avalon and its Subsidiaries have not effected any securitization transactions or “off-balance sheet arrangements” (as defined in Item 303(c) of Regulation S-K of the SEC) since Avalon Audit Date. Avalon has delivered or made available to Sen Lang copies of the documentation creating or governing all such all securitization transactions and off-balance sheet arrangements.
4.16 Loans to Executives and Directors. Avalon has not, since the effective date of SOX, extended or maintained credit, arranged for the extension of credit, or renewed an extension of credit, in the form of a personal loan to or for any director or executive officer (or equivalent thereof) of Avalon in violation of SOX. Avalon has not made any loan or extension of credit to which the second sentence of Section 13(k)(I) of the Exchange Act applies.
4.17 Independent Auditors. Marcum LLP serves as Avalon’s independent registered public accounting firm and to Avalon’s knowledge, there are no relationships or services, or any other factors that may affect the objectivity and independence of Marcum LLP under applicable auditing standards. Marcum LLP has not performed any non-audit services for Avalon and its Subsidiaries since the Avalon Audit Date, which, in any such case, were required to be disclosed in Avalon SEC Reports and were not so disclosed.
A-26
4.18 Absence of Changes or Events. Except for (a) matters publicly disclosed by Avalon prior to the date hereof in Avalon SEC Reports filed prior to the date hereof, (b) matters disclosed in Section 4.18 of the Avalon Disclosure Schedule and (c) matters disclosed in Section 4.21 of the Avalon Disclosure Schedule:
4.18.1 Since December 31, 2019, other than in the ordinary course of business consistent with past practice or as disclosed in the Avalon SEC Reports : (i) Avalon and its Subsidiaries have conducted their business in the ordinary course and have not entered into any material oral or written agreement or other material transaction that is not in the ordinary course of business (other than this Agreement) or that could reasonably be expected to result in an Avalon Material Adverse Effect; (ii) neither Avalon nor any of its Subsidiaries have sustained any material loss or interference with their business or properties from fire, flood, windstorm, accident, strike or other calamity (whether or not covered by insurance); (iii) there has been no material change in the indebtedness of Avalon and its Subsidiaries, no change in the capital stock of Avalon and no dividend or distribution of any kind declared, paid or made by Avalon on any class of its capital stock; (iv) there has been no event or condition which has caused an Avalon Material Adverse Effect, nor any development, occurrence or state of facts or circumstances known to Avalon that could, singly or in the aggregate, reasonably be expected to result in an Avalon Material Adverse Effect; and (v) there has been no material change by Avalon in its accounting principles, practices or methods.
4.18.2 Since December 31, 2019, other than in the ordinary course of business consistent with past practice or as disclosed in the Avalon SEC Reports, there has not been any increase in the compensation or other benefits payable, or which could become payable, by Avalon, to its officers or key employees, or any amendment of any of the Avalon Employee Benefit Plans.
4.19 Capitalization.
4.19.1 Subject to Section 4.19.1 of the Avalon Disclosure Schedule, the authorized capital stock of Avalon consists solely of 490,000 shares of Avalon Common Stock, and 10,000 shares of preferred stock, without par value (the “Avalon Preferred Stock”). As of March 31, 2021, there were 84,425,564 shares of Avalon Common Stock and zero (0) shares of Avalon Preferred Stock outstanding. Except for the foregoing and as disclosed in the Avalon SEC Reports, there are not any existing options, warrants, calls, subscriptions, or other rights or other agreements or commitments obligating Avalon to issue, transfer or sell any shares of capital stock of Avalon or any other securities convertible into or evidencing the right to subscribe for any such shares. There are no outstanding stock appreciation rights with respect to the capital stock of Avalon. As of the date hereof, except for (a) stock options issuable pursuant to stock option plans adopted or assumed by Avalon, (b) shares of Avalon Common Stock issuable pursuant to other Avalon Employee Benefit Plans disclosed in Avalon SEC Reports, (c) securities issuable in connection with business combinations disclosed in Avalon SEC Reports and (d) matters described in Section 4.19.1 of the Avalon Disclosure Schedule, Avalon is not bound by any outstanding subscriptions, options, warrants, calls, commitments or agreements of any character calling for the purchase or issuance of any shares of Avalon Common Stock or Avalon Preferred Stock or any other equity securities of Avalon or any securities representing the right to purchase or otherwise receive any shares of Avalon Common Stock or Avalon Preferred Stock or any other equity securities of Avalon.
A-27
4.19.2 Except as set forth in Section 4.19.2 of the Avalon Disclosure Schedule, there are no (i) obligations, contingent or otherwise, of Avalon or its Subsidiaries to repurchase, redeem or otherwise acquire any shares of Avalon Common Stock or provide funds to, or make any investment in (in the form of a loan, capital contribution or otherwise), or provide any guarantee with respect to the obligations of, any other person, or (ii) agreements, arrangements or commitments of any character (contingent or otherwise) pursuant to which any person is or may be entitled to receive any payment based on the revenues or earnings (or any component thereof), or calculated in accordance therewith, of Avalon or any of its Subsidiaries. There are no voting trusts, proxies or other agreements or understandings to which Avalon is a party or by which Avalon is bound with respect to the voting of any shares of capital stock of Avalon.
4.19.3 Avalon has delivered or made available to Sen Lang complete and correct copies of each stock option plan adopted or assumed by Avalon as of the date hereof.
4.19.4 Each outstanding share of Avalon Common Stock and Avalon Preferred Stock is, and all shares of Avalon Common Stock to be issued in connection with the transactions contemplated hereby will be, duly authorized and validly issued, fully paid and nonassessable, with no personal liability attaching to the ownership thereof, and each outstanding share of Avalon Common Stock and Avalon Preferred Stock has not been, and all shares of Avalon Common Stock to be issued in connection with the transactions contemplated hereby will not be, subject to or issued in violation of any preemptive or similar rights.
4.20 Capital Stock of Subsidiaries. The only direct or indirect Subsidiaries of Avalon are those listed in Section 4.20 of the Avalon Disclosure Schedule. Avalon is directly or indirectly the record and beneficial owner of all of the outstanding shares of capital stock of each of its Subsidiaries, there are no proxies with respect to such shares, and there are not any existing options, warrants, calls, subscriptions, or other rights or other agreements or commitments obligating Avalon or any of such Subsidiaries to issue, transfer or sell any shares of capital stock of any of such Subsidiaries or any other securities convertible into or evidencing the right to subscribe for any such shares. All of such shares so beneficially owned by Avalon are duly authorized and validly issued, fully paid, nonassessable and free of preemptive rights with respect thereto and are owned by Avalon, directly or indirectly, free and clear of any claim, lien or encumbrance of any kind with respect thereto. Except as set forth in Section 4.20 of the Avalon Disclosure Schedule, Avalon does not directly or indirectly own any interest in any corporation, partnership, limited liability company, joint venture or other business association or entity.
4.21 Litigation. Except as set forth in Section 4.21 of the Avalon Disclosure Schedule or in the Avalon SEC Reports, as of the date hereof there are no material pending Actions or, to the knowledge of Avalon, investigations by, against or affecting Avalon, any of its Subsidiaries or any of their properties, assets or operations, or with respect to which Avalon or any of its Subsidiaries is responsible by way of indemnity or otherwise. Except as set forth in Section 4.21 of the Avalon Disclosure Schedule or the Avalon SEC Reports: (i) no material pending or, to the knowledge of Avalon, threatened Actions or investigations by, against or affecting Avalon, any of its Subsidiaries or any of their properties, assets or operations, or with respect to which they are responsible by way of indemnity or otherwise, whether or not disclosed in such Avalon SEC Reports, would, singly or in the aggregate with all such other Actions or investigations, reasonably be expected to have an Avalon Material Adverse Effect; and (ii) to the knowledge of Avalon, there are no material Actions or investigations and there is no reasonable basis, to the knowledge of Avalon, for any Action or investigation, whether or not threatened or contemplated.
A-28
4.22 Insurance. Avalon and its Subsidiaries have insurance policies and fidelity bonds covering it and its Subsidiaries’ assets, business, equipment, properties, operations, employees, officers and directors which Avalon reasonably and in good faith believes are adequate to conduct the business of Avalon and its Subsidiaries. All premiums due and payable under all such policies and bonds have been paid, and Avalon is otherwise in full compliance with the terms and conditions of all such policies and bonds, except where the failure to have made payment or to be in full compliance would not, individually or in the aggregate with all such other failures, have an Avalon Material Adverse Effect. Avalon reasonably believes that the reserves established by Avalon and its Subsidiaries in respect of all matters as to which Avalon or any of its Subsidiaries self-insures or carries retention and/or deductibles, including workers’ medical coverage and workers’ compensation, are adequate and appropriate, and Avalon is not aware of any facts or circumstances existing as of the date hereof that would reasonably be expected to cause such reserves to be materially inadequate or inappropriate.
4.23 Title to and Condition of Properties. Avalon and its Subsidiaries have good title to all of the real property and personal property reflected on Avalon’s December 31, 2020 unaudited consolidated balance sheet contained in Avalon’s Quarterly Report on Form 10-K for the year ended December 31, 2020 filed with the SEC (the “Avalon Balance Sheet”), except for property since sold or otherwise disposed of in the ordinary course of business and consistent with past practice and except for defects of title which are not material to Avalon and its Subsidiaries taken as a whole. Neither Avalon nor any of its Subsidiaries owns any material real property. No real or tangible personal property owned or leased by Avalon or any of its Subsidiaries is subject to claims, liens or other Encumbrances of any kind or character, including mortgages, pledges, liens, conditional sale agreements, charges, security interests, easements, restrictive covenants, rights of way or options, except for (i) Encumbrances for Taxes not yet delinquent or which are being contested in good faith by appropriate Action and in respect of which Avalon or its appropriate Subsidiary has set aside on its books adequate reserves in accordance with generally accepted accounting principles; (ii) mechanics’, carriers’, workers’, repairers’, materialmen’s, landlords’ and other similar statutory or common law liens incurred in the ordinary course of business for obligations not yet delinquent or the validity of which is being contested in good faith by appropriate Actions and in respect of which Avalon or its appropriate Subsidiary has set aside on its books adequate reserves in accordance with generally accepted accounting principles; (iii) in the case of real property, easements, rights of way, restrictions, minor defects or irregularities in title that do not individually or in the aggregate have a material adverse effect on the value or use of the real property encumbered thereby as currently used in the operation of the business of Avalon or its Subsidiaries; (iv) those which would not materially interfere with the conduct of the business of Avalon and its Subsidiaries (the encumbrances described in clauses (i) through (iv) of this sentence, collectively, the “Avalon Permitted Encumbrances”); (v) those securing liabilities reflected in the Avalon Balance Sheet; or (vi) those described in Section 4.23 of the Avalon Disclosure Schedule.
A-29
4.24 Leases. There have been delivered or made available to Sen Lang true and complete copies of each lease pursuant to which Real Property or personal property is held under lease by Avalon or any of its Subsidiaries (limited, in the case of personal property, to leases pursuant to which annual rentals are reasonably expected to be at least $100,000 per year), and true and complete copies of each lease pursuant to which Avalon or any of its Subsidiaries leases real or personal property to others (limited in the case of personal property, to leases pursuant to which annual rentals are reasonably expected to be at least $100,000 per year). Section 4.24 of the Avalon Disclosure Schedule sets forth a true and complete list of all such leases, and such leases are the only leases that are material to the business conducted by Avalon and its Subsidiaries taken as a whole. All of the leases so listed (i) are, in all material respects, valid and subsisting and in full force and effect with respect to Avalon and its Subsidiaries, as the case may be, and, to Avalon’s knowledge, with respect to any other party thereto and (ii) were entered into as a result of bona fide arm’s length negotiations with the other party or parties thereto. Avalon or its Subsidiaries, as the case may be, have valid leasehold interests in all properties leased thereunder free and clear of all material liens and encumbrances other than Avalon Permitted Encumbrances. The real properties leased by Avalon and its Subsidiaries are, in all material respects, in good operating order and condition, subject to ordinary wear and tear. To Avalon’s knowledge, there are no material structural, mechanical or other defects in any improvements located on such real properties.
4.25 Contracts and Commitments. Except as set forth in Section 4.25 of the Avalon Disclosure Schedule or as set forth as an exhibit in an Avalon SEC Report filed since December 31, 2019, neither Avalon nor any of its Subsidiaries is a party to any existing contract, obligation or commitment of any type in any of the following categories:
4.25.1 contracts for the purchase by Avalon or any of its Subsidiaries of medicines, materials, supplies or equipment which are not cancelable upon ninety (90) days’ or less notice and which either (i) have not been entered into in the ordinary course of business and consistent with past practice or (ii) provide for purchase prices substantially greater than those presently prevailing for such materials, supplies or equipment, or (iii) contracts obligating Avalon or its Subsidiaries to make capital expenditures in excess of $50,000;
4.25.2 contracts under which Avalon or any of its Subsidiaries has, except by way of endorsement of negotiable instruments for collection in the ordinary course of business and consistent with past practice, become absolutely or contingently or otherwise liable for (i) the performance of any other person, firm or corporation under a contract, or (ii) the whole or any part of the indebtedness or liabilities of any other person, firm or corporation;
4.25.3 powers of attorney outstanding from Avalon or any of its Subsidiaries other than as issued in the ordinary course of business and consistent with past practice with respect to customs, insurance, patent, trademark or tax matters, or to agents for service of process;
4.25.4 contracts under which any amount payable by Avalon or any of its Subsidiaries is dependent upon, or calculated in accordance with, the revenues or earnings (or any component thereof of Avalon or any of its Subsidiaries;
4.25.5 contracts with any director, officer, employee or affiliate of Avalon or any of its Subsidiaries other than in such person’s capacity as a director, officer or employee of Avalon or any of its Subsidiaries;
4.25.6 contracts which limit or restrict where Avalon or any of its Subsidiaries may conduct its business or the type or line of business in which Avalon or any of its Subsidiaries may engage;
4.25.7 contracts with any party for the loan of money or availability of credit to or from Avalon or any of its Subsidiaries (except credit extended by Avalon or any of its Subsidiaries to its customers in the ordinary course of business and consistent with past practice);
A-30
4.25.8 any material hedging, option, derivative or other similar transaction; or
4.25.9 contracts pursuant to which Avalon or any of its Subsidiaries (a) grants any Person any exclusive license, exclusive option or other exclusive right with respect to any Avalon Intellectual Property, (b) grants any Person any license, option or other right with respect to any Avalon Intellectual Property that is material to the business of Avalon and its Subsidiaries or (c) is granted any license, option or other right with respect to any Avalon Intellectual Property that is material to the business of Avalon and its Subsidiaries.
True and complete copies of all contracts, obligations and commitments listed in Section 4.25 of the Avalon Disclosure Schedule have been delivered or made available to Sen Lang. None of Avalon or its Subsidiaries or, to the knowledge of Avalon, any other party is in breach of or default under any of the contracts, obligations and commitments listed in Section 4.25 of the Avalon Disclosure Schedule or under any other Avalon Contracts (and, to the knowledge of Avalon, no facts or circumstances exist which could reasonably support the assertion of any such breach or default) except for breaches and defaults which would not, singly or in the aggregate with all other such breaches, have an Avalon Material Adverse Effect.
4.26 Employees; Labor Matters. Except as set forth in Section 4.26 of the Avalon Disclosure Schedule, neither Avalon nor any Subsidiary thereof is a party to or bound by any collective bargaining agreement, and there are no labor unions or other organizations representing, purporting to represent or attempting to represent any employees employed by Avalon or any Subsidiary thereof. Since December 31, 2019, there has not occurred or been threatened any material strike, slowdown, picketing, work stoppage, concerted refusal to work overtime or other similar labor activity with respect to any employees of Avalon or any Subsidiary thereof. Except as set forth in Section 4.26 of the Avalon Disclosure Schedule, there are no labor disputes currently subject to any grievance procedure, arbitration or litigation and there is no representation petition pending or threatened with respect to any employee of Avalon or any Subsidiary thereof. Each of Avalon and its Subsidiaries has complied with Applicable Law pertaining to the employment or termination of employment of their respective employees, including Applicable Law relating to labor relations, equal employment opportunities, fair employment practices, prohibited discrimination or distinction and other similar employment activities, except for any failure so to comply that, individually and in the aggregate, could not result in any material liability or obligation on the part of Avalon or any of its Subsidiaries.
4.27 Put Rights. Except as described in Section 4.27 of the Avalon Disclosure Schedule, neither the execution and delivery by Avalon of this Agreement nor the consummation of the Acquisition or any other transaction contemplated hereby gives rise to any obligation of Avalon or any of its Subsidiaries to, or any right of any holder of any security of Avalon or any of its Subsidiaries to require Avalon to, purchase, offer to purchase, redeem or otherwise prepay or repay any such security, or deposit any funds to effect the same.
4.28 Employment and Labor Contracts. Except as set forth in Section 4.28 of the Avalon Disclosure Schedule, neither Avalon nor any of its Subsidiaries is a party to any employment, management services, consultation or other contract or agreement with any past or present officer, director or employee or, to the knowledge of Avalon, any entity affiliated with any past or present officer, director or employee, other than the agreements executed by employees generally, the forms of which have been provided to Sen Lang.
A-31
4.29 Intellectual Property Rights. Avalon or its Subsidiaries own or have the right to use all Avalon Intellectual Property Rights (as defined herein) necessary to the conduct of their respective businesses. Subject to obtaining any associated consents with respect to agreements or licenses listed in Section 4.29 of the Avalon Disclosure Schedule, each Avalon Intellectual Property Right owned or used by Avalon or any of its Subsidiaries immediately prior to the Closing will be owned or available for use by Avalon or its Subsidiaries on substantially the same terms and conditions immediately subsequent to the Closing. Section 4.29 of the Avalon Disclosure Schedule contains a list of all patents, trade names, registered copyrights, trademarks and service marks, mask works and applications for the foregoing owned by Avalon or its Subsidiaries. Except as set forth in Section 4.29 of the Avalon Disclosure Schedule, (i) Avalon and/or its Subsidiaries have sole and exclusive ownership of, and valid and unencumbered (except for Avalon Permitted Encumbrances) title to, the Avalon Intellectual Property Rights set forth in such Section 4.29 and, to Avalon’s knowledge, such title has not been challenged (pending or threatened) by others except for the encumbrances listed therein; (ii) there have been no claims or assertions made by others that Avalon or its Subsidiaries has infringed or misappropriated any Intellectual Property Rights of others by the development, manufacture or sale of products, the rendering of services or any other activity since December 31, 2016; (iii) to the knowledge of Avalon, there has been no such infringement or misappropriation by Avalon or any of its Subsidiaries since December 31, 2019; (iv) Avalon has no knowledge of any infringement or misappropriation of Avalon Intellectual Property Rights of Avalon or any of its Subsidiaries by others; and (v) all Avalon Intellectual Property Rights owned by Avalon or its Subsidiaries are in good standing with the registration authority therefore, if any, and, to the extent recorded on the public record, are recorded in the name of Avalon or its Subsidiaries. True and complete copies of all material listed in Section 4.29 of the Avalon Disclosure Schedule have been delivered or made available to Sen Lang.
4.30 Taxes.
4.30.1 Except as set forth in Section 4.30.1 in the Avalon Disclosure Schedule, (i) all Returns required to be filed by, or with respect to any activities or assets of, each of Avalon and its Subsidiaries have been duly and timely filed and are correct and complete in all material respects, and (ii) all Taxes shown as owing on such Returns have been paid.
4.30.2 Except as set forth in Section 4.30.2 of the Avalon Disclosure Schedule, none of Avalon nor any of its Subsidiaries have waived any statute of limitations in respect of Taxes or agreed to any extension of time with respect to a Tax assessment or deficiency.
4.30.3 Except as set forth in Section 4.30.3 of the Avalon Disclosure Schedule, none of Avalon nor any of its Subsidiaries are a party to any Tax allocation or sharing agreement.
4.30.4 Section 4.30.4 of the Avalon Disclosure Schedule lists all Income Tax Returns that have been filed with respect to each of Avalon and its Subsidiaries for taxable periods ended on or after December 31, 2018 and that have not yet been audited or are currently the subject of audit.
A-32
4.30.5 Except as set forth in Section 4.30.5 of the Avalon Disclosure Schedule, none of Avalon or its Subsidiaries (i) is a party to or bound by or has any obligation under any Tax allocation, sharing, indemnity or similar agreement or arrangement or (ii) is or has been a member of any group of companies filing a consolidated, combined or unitary Income Tax Return.
4.31 Employee Benefit Plans.
4.31.1 Except as set forth in Section 4.31 of the Avalon Disclosure Schedule, with respect to any employee or former employee of Avalon or any Subsidiary thereof, none of Avalon or any Subsidiary thereof, or any affiliated company presently maintains, contributes to or has any liability under: (i) any bonus, incentive compensation, profit sharing, retirement, pension, group insurance, death benefit, cafeteria, medical expense reimbursement, dependent care, stock option, stock purchase, stock appreciation rights, deferred compensation, consulting, severance pay or termination pay, vacation pay, welfare or other employee benefit or fringe benefit plan, program or arrangement; or (ii) any plan, program or arrangement which is an employee pension benefit plan, or an “employee welfare benefit plan” as defined under relevant laws. Each plan, program and arrangement set forth in Section 4.31 of the Avalon Disclosure Schedule is herein referred to as a “Avalon Employee Benefit Plan.” The term “affiliated company” means any organization that would be aggregated with any of Avalon or any Subsidiary thereof under Section 414(b), (c), (m) or (o) of the Code.
4.31.2 There is no pending or threatened legal Action or investigation against or involving any Avalon Employee Benefit Plan (other than routine claims for benefits) and there is no basis for any facts which could give rise to any such Action or investigation.
4.31.3 None of Avalon or any Subsidiary thereof nor any of its Affiliates is a party to any employment agreement, whether written or oral, or agreement with change in control or similar provisions, or a collective bargaining agreement or contract with any labor union relating to any employees or former employees of Avalon or any Subsidiary thereof.
4.32 Environmental Matters.
4.32.1 Each of Avalon and its Subsidiaries has complied and is in compliance in all material respects with all applicable Environmental Laws pertaining to any of the properties and assets of Avalon and its Subsidiaries (including all real property owned by Avalon or any of its Subsidiaries, together with all structures, facilities, improvements, fixtures, systems, equipment and items of property presently or hereafter located thereon or attached or appurtenant thereto or owned by Avalon or any of its Subsidiaries and located on real property leased by Avalon or any of its Subsidiaries, and all easements, licenses, rights and appurtenances relating to the foregoing (collectively the “Avalon Real Property”)) and the use and ownership thereof, and to the operation of their respective businesses. No material violation by Avalon or any of its Subsidiaries is being alleged of any applicable Environmental Law relating to any of the properties and assets of Avalon or any of its Subsidiaries including (the Avalon Real Property) or the use or ownership thereof, or to the operation of their respective businesses.
A-33
4.32.2 None of Avalon or its Subsidiaries or any other Person (including any tenant or subtenant) has caused or taken any action that will result in, nor is Avalon or any Subsidiary thereof subject to, any material liability or obligation on the part of Avalon or any Subsidiary thereof or any of its Affiliates, relating to (x) the environmental conditions on, under, or about the Avalon Real Property or other properties or assets owned, leased, operated or used by Avalon or any of its Subsidiaries or any predecessor thereto at the present time or in the past, including the air, soil and groundwater conditions at such properties or (y) the past or present use, management, handling, transport, treatment, generation, storage, disposal or Release of any Hazardous Materials (as defined herein).
4.32.2.1 Avalon has disclosed and made available to the Sen Lang all information, including all studies, analyses and test results, in the possession, custody or control of or otherwise known to Avalon relating to (x) the environmental conditions on, under or about the Real Property or other properties or assets owned, leased, operated or used by Avalon or any of its Subsidiaries any predecessor in interest thereto at the present time or in the past, and (y) any Hazardous Materials used, managed, handled, transported, treated, generated, stored or Released by Avalon or any of its Subsidiaries or any other Person on, under, about or from any of the Avalon Real Property, or otherwise in connection with the use or operation of any of the properties and assets of Avalon or any of its Subsidiaries or their respective businesses.
ARTICLE V.
COVENANTS OF THE PARTIES
5.1 Access and Information.
5.1.1 Prior to the Closing, Avalon shall be entitled to make or cause to be made such investigation of the Sen Lang Parties, the Acquired Companies, and the financial and legal condition thereof, as Avalon deems necessary or advisable, and Sen Lang shall cooperate with any such investigation. In furtherance of the foregoing, but not in limitation thereof, Sen Lang shall (a) permit Avalon and its agents and representatives or cause them to be permitted to have full and complete access to the premises, operating systems, computer systems (hardware and software) and books and records of the Acquired Companies upon reasonable notice during regular business hours, (b) furnish or cause to be furnished to Avalon such financial and operating data, projections, forecasts, business plans, strategic plans and other data relating to the Acquired Companies and their businesses as Avalon shall request from time to time, and (c) cause its accountants to furnish to Avalon and its accountants access to all work papers relating to any of the periods covered by financial statements provided by the Acquired Companies to Avalon hereunder.
5.1.2 Prior to the Closing, and except for disclosures which would cause Avalon or any of its Subsidiaries to waive the attorney-client privilege or otherwise violate Applicable Law or any material confidentiality agreement, Avalon shall provide complete and accurate information to the Sen Lang Representative and its representatives in response to reasonable requests for information made in order to enable the Sen Lang Representative to confirm the accuracy of the representations set forth in ARTICLE IV (including the continuing accuracy of those representations which are not made as of a particular date) and the fulfillment of the covenants of this ARTICLE V and the closing conditions in Sections 6.1 and 6.3.
A-34
5.1.3 Prior to the Closing, no Party shall use any information provided to it in confidence by another Party for any purposes unrelated to this Agreement. Except with respect to publicly available documents, in the event that this Agreement is terminated, (a) Avalon will return to the Sen Lang Representative all documents obtained by it from the Acquired Companies in confidence and any copies thereof in the possession of Avalon or its agents and representatives or, at the option of Avalon, Avalon shall cause all of such documents and all of such copies to be destroyed and shall certify the destruction thereof to the Sen Lang Representative and (b) the Sen Lang Owners will return to Avalon all documents obtained by it from Avalon and its Subsidiaries in confidence and any copies thereof in the possession of any Acquired Company or their agents and representatives or, at the option of the Sen Lang Representative, the Sen Lang Owners shall cause all of such documents and all of such copies to be destroyed and shall certify the destruction thereof to Avalon. No investigation by any Party heretofore or hereafter made shall modify or otherwise affect the conditions to the obligation of such Party to consummate the transactions contemplated hereby.
5.2 Sen Lang’s Affirmative Covenants. Prior to the Closing, except as otherwise expressly provided herein, the Sen Lang Parties shall (and the Sen Lang Parties shall cause each of the Acquired Companies to):
5.2.1 conduct its business only in the ordinary and regular course of business consistent with past practices;
5.2.2 conduct its business activities within the permitted scope of business or is otherwise operating its business in full compliance with all relevant legal requirements and with all requisite approvals granted by competent PRC authorities;
5.2.3 use commercially reasonable efforts to keep in full force and effect its corporate existence and all material rights, franchises, the Sen Lang Intellectual Property Rights and goodwill relating or pertaining to its businesses;
5.2.4 endeavor to retain its employees and preserve its present relationships with customers, suppliers, contractors, distributors and employees, and continue to compensate its employees consistent with past practices;
5.2.5 maintain its other assets in customary repair, order and condition and maintain insurance reasonably comparable to that in effect on the date of this Agreement;
5.2.6 maintain its books, accounts and records in accordance with generally accepted accounting principles;
5.2.7 use commercially reasonable efforts to obtain all authorizations, consents, waivers, approvals or other actions and to make all filings and applications necessary or desirable to consummate the transactions contemplated hereby and to cause the other conditions to Avalon’s obligation to close to be satisfied; and
A-35
5.2.8 promptly notify Avalon in writing if, prior to the consummation of the Closing, to its knowledge (a) any of the representations and warranties contained in Article III cease to be accurate and complete in all material respects (except for any representation and warranty (i) which is qualified hereunder as to materiality, as to which such notification shall be given if any Acquired Company obtains knowledge that such representation and warranty is inaccurate in any respect, or (ii) that addresses matters only as of a particular date, which need only be true and correct as of such date) or (b) Sen Lang fails to comply with or satisfy any material covenant, condition or agreement to be complied with or satisfied by it hereunder; provided, however, that the delivery of any notice pursuant to this Section 5.2.8 shall not limit or otherwise affect the remedies available hereunder to Avalon.
5.3 Avalon’s Affirmative Covenants. Prior to the Closing, except as otherwise expressly provided herein, Avalon shall (and Avalon shall cause each of its Subsidiaries to):
5.3.1 use commercially reasonable efforts to keep in full force and effect its corporate existence and all material rights, franchises, the Avalon Intellectual Property Rights and goodwill relating or obtaining to its businesses;
5.3.2 endeavor to retain its employees and preserve its present relationships with customers, suppliers, contractors, distributors and employees;
5.3.3 maintain its books, accounts and records in accordance with generally accepted accounting principles;
5.3.4 use commercially reasonable efforts to obtain all authorizations, consents, waivers, approvals or other actions and to make all filings and applications necessary or desirable to consummate the transactions contemplated hereby and to cause the other conditions to Sen Lang’s obligation to close to be satisfied; and
5.3.5 promptly notify Sen Lang in writing if, prior to the consummation of the Closing, to its knowledge (a) any of the representations and warranties contained in Article IV cease to be accurate and complete in all material respects (except for any representation and warranty (i) which is qualified hereunder as to materiality, as to which such notification shall be given if Avalon or its Subsidiaries obtain knowledge that such representation and warranty is inaccurate in any respect, or (ii) that addresses matters only as of a particular date, which need only be true and correct as of such date) or (b) Avalon fails to comply with or satisfy any material covenant, condition or agreement to be complied with or satisfied by it hereunder; provided, however, that the delivery of any notice pursuant to this Section 5.3.5 shall not limit or otherwise affect the remedies available hereunder to Sen Lang; and
5.3.6 cause the consolidated balance sheets and the related consolidated statements of income and cash flows (including the related notes thereto) of Avalon included in Avalon SEC Reports filed after the date hereof to comply, in all material respects, with applicable accounting requirements and the published rules and regulations of the SEC with respect thereto, to be prepared, in accordance with U.S. generally accepted accounting principles applied on a basis consistent with prior periods (except as otherwise noted therein), and to present fairly, in all material respects, the consolidated financial position of Avalon and its consolidated Subsidiaries as of their respective dates, and the consolidated results of their operations and their cash flows for the periods presented therein (subject, in the case of the unaudited interim financial statements, to notes and normal year-end adjustments that are not reasonably expected to be, material in amount or effect).
A-36
5.4 Sen Lang’s Negative Covenants. Prior to the Closing, without the prior written consent of Avalon or as otherwise expressly provided herein, the Sen Lang Parties will not, and the Sen Lang Parties will cause each Acquired Company not to:
5.4.1 take any action or omit to take any action which would result in any Acquired Company (a) incurring any trade accounts payable outside of the ordinary course of Business or making any commitment to purchase quantities of any item of inventory in excess of quantities normally purchased in the ordinary course of business; (b) increasing any of its indebtedness for borrowed money except in the ordinary course of business; (c) guaranteeing the obligations of any entity other than an Acquired Company, (d) making any purchases of pharmaceuticals other than from the manufacturers thereof or wholesalers or other distributors authorized by such manufacturers to distribute their products; (e) merging or consolidating with, purchasing substantially all of the assets of, or otherwise acquiring any business or any proprietorship, firm, association, limited liability company, corporation or other business organization; (f) increasing or decreasing the rate or type of compensation payable to any officer, director, employee or consultant of any Acquired Company (other than regularly scheduled increases in base salary and annual bonuses consistent with prior practice); (g) entering into or amending any collective bargaining agreement, or creating or modifying any pension or profit-sharing plan, bonus, deferred compensation, death benefit, or retirement plan, or any other employee benefit plan, or increasing the level of benefits under any such plan, or extending the exercisability of any outstanding stock option or increasing or decreasing any severance or termination pay benefit or any other fringe benefit; (h) making any representation to anyone indicating any intention of Avalon or its Subsidiaries to retain, institute, or provide any employee benefit plans; (i) declaring or paying any dividend or making any distribution with respect to, or purchasing or redeeming, shares of the capital stock of any Acquired Company; (j) selling, licensing or disposing of any assets otherwise than in the ordinary course of business of the Acquired Companies; (k) making any capital expenditures other than in the ordinary course of business consistent with past practices and in no event in excess of $50,000 in the aggregate; (l) issuing any shares of the capital stock of any kind of any Acquired Company, transferring from the treasury of any Acquired Company any shares of the capital stock of any Acquired Company or issuing or granting any subscriptions, options, rights, warrants, convertible securities or other agreements or commitments to issue, or contracts or any other agreements obligating any Acquired Company to issue, or to transfer from treasury, any shares of capital stock of any class or kind, or securities convertible into any such shares; (m) modifying, amending or terminating any material Sen Lang Contract other than in the ordinary course of business that is consistent with past practices; or (n) entering into any other transaction outside of the ordinary course of business;
5.4.2 change any method or principle of accounting in a manner that is inconsistent with past practice, except to the extent required by generally accepted accounting principles as advised by Sen Lang’s regular independent accountants;
5.4.3 make, change or revoke any material Tax election, fail to pay any income or other material Tax as such Tax becomes due and payable, file any amendment making any material change to any Tax Return, settle or compromise any income or other material Tax liability, enter into any Tax allocation, sharing, indemnification or other similar agreement or arrangement, request or consent to any extension or waiver of any limitation period with respect to any claim or assessment for any income or other material Taxes (other than in connection with any extension of time to file any Tax Return), or adopt or change any accounting method in respect of Taxes;
A-37
5.4.4 take any action that would likely result in the representations and warranties set forth in Article III (other than representations made as of a particular date) becoming false or inaccurate in any material respect (or, as to representations and warranties, which, by their terms, are qualified as to materiality, becoming false or inaccurate in any respect);
5.4.5 incur or create any encumbrances, liens, pledges or security interests on assets other than Sen Lang Permitted Encumbrances;
5.4.6 except as contemplated herein, take any action or omit to take any action which would materially interfere with Avalon’s rights to compel performance of each of the obligations of Sen Lang under this Agreement;
5.4.7 take or omit to be taken any action, or permit any of its affiliates to take or to omit to take any action, which would reasonably be expected to result in a Sen Lang Material Adverse Effect; or
5.4.8 agree or commit to take any action precluded by this Section 5.4.
5.5 Avalon’s Negative Covenants. Prior to the Closing, without the prior written consent of Sen Lang or as otherwise expressly provided herein, Avalon will not and Avalon will cause its Subsidiaries not to:
5.5.1 change any method or principle of accounting in a manner that is inconsistent with past practice, except to the extent required by generally accepted accounting principles as advised by Avalon’s regular independent accountants;
5.5.2 take any action that would likely result in the representations and warranties set forth in ARTICLE IV (other than representations made as of a particular date) becoming false or inaccurate in any material respect (or, as to representations and warranties, which, by their terms, are qualified as to materiality, becoming false or inaccurate in any respect);
5.5.3 except as contemplated herein, take any action or omit to take any action which would materially interfere with Sen Lang’s rights to compel performance of each of the obligations of Avalon under this Agreement;
5.5.4 take or omit to be taken any action, or permit any of its affiliates to take or to omit to take any action, which would reasonably be expected to result in an Avalon Material Adverse Effect;
5.5.5 amend Avalon’s certificate of incorporation or by-laws, each as amended, in any material manner that does not generally apply to all of Avalon’s stockholders; or
A-38
5.5.6 agree or commit to take any action precluded by this Section 5.5.
Notwithstanding any provision to the contrary contained in this Agreement, (i) in no event shall Avalon be prohibited from merging or consolidating with, purchasing substantially all of the assets of, or otherwise acquiring, and Avalon is expressly permitted to, merge or consolidate with, purchase substantially all of the assets of, or otherwise acquire, any business or any proprietorship, firm, association, limited liability company, corporation or other business organization; (ii) in no event shall Avalon be prohibited from effecting, and Avalon is expressly permitted to effect, a stock split, reclassification, combination or other change with respect to shares of Avalon Common Stock; provided, that the Exchange Stocks of each Sen Lang Shareholder set forth on Exhibit A hereto shall be adjusted to reflect any such stock split, reclassification, combination or other change; and (iii) in no event shall Avalon be prohibited from cancelling any and all outstanding out-of-the-money options and/or warrants to purchase shares of Avalon Common Stock and issuing to the holders of such cancelled options and/or warrants, options and/or warrants purchase shares of the Avalon Common Stock on the same terms at an exercise price equal to the per share closing price of the Avalon Common Stock reported on Nasdaq on the date of such issuance, and Avalon is expressly permitted to cancel and issue any such options and/or warrants.
5.6 Exclusive Dealing. The Sen Lang Parties will not, and will direct their Affiliates and Representatives not to, (i) solicit, initiate, or encourage the submission of any proposal or offer from any Person relating to the acquisition of any capital stock or other voting securities, or any assets (excluding sales of inventory in the ordinary course of business), of the Acquired Companies (including any acquisition structured as a merger, consolidation, or share exchange), (ii) enter into or renew any distribution agreement related to the business of the Acquired Companies or (iii) participate in any discussions, conversations, negotiations or other communications regarding, or furnish to any Person, any information with respect to, or otherwise cooperate in any way, assist or participate in, facilitate or encourage any effort or attempt by any other Person to seek to do any of the foregoing. The Acquired Companies immediately shall cease and cause to be terminated all existing discussions, conversations, negotiations and other communications with any Persons conducted heretofore with respect to any of the foregoing. The Sen Lang Representative shall notify Avalon promptly, but in any event within twenty-four (24) hours, if any Person makes any such proposal or offer. No Acquired Company shall release any Person from, or waive any provision of, any confidentiality agreement to which any Acquired Company is a party, without the prior written consent of Avalon.
5.7 Closing Documents. The Sen Lang Parties shall, prior to or on the Closing Date, execute and deliver, or cause to be executed and delivered, to Avalon the documents or instruments described in Section 6.2. Avalon shall, prior to or on the Closing Date, execute and deliver, or cause to be executed and delivered, to the Sen Lang Representative the documents or instruments described in Section 6.3.
A-39
5.8 Further Actions. Each of the parties hereto agrees to use all reasonable efforts to take, or cause to be taken, all actions, and to do, or cause to be done, and to assist and cooperate with the other parties in doing, all things necessary, proper or advisable to consummate and make effective, in the most expeditious manner practicable in light of the circumstances, the Acquisition and the other transactions contemplated by this Agreement, including (A) the obtaining of all other necessary actions or nonactions, waivers, consents, licenses, permits, authorizations, orders and approvals from Governmental Authorities and the making of all other necessary registrations and filings, (B) the obtaining of all consents, approvals or waivers from third parties related to or required in connection with the Acquisition that are necessary to consummate the Acquisition and the transactions contemplated by this Agreement or required to prevent an Avalon Material Adverse Effect or a Sen Lang Material Adverse Effect from occurring prior to or after the Closing, (C) the preparation of the Proxy Statement and the mailing of the Proxy Statement to the stockholders of Avalon and the Sen Lang Shareholders, (D) if necessary as a result of the circumstances, the amendment of the Proxy Statement as required by law and (E) the execution and delivery of any additional instruments necessary to consummate the transactions contemplated by, and to fully carry out the purposes of, this Agreement.
5.9 Public Announcements. Unless otherwise required by Applicable Law or requirements of Nasdaq (and in that event only if time does not permit), the Parties (including, at and after the Closing, the Surviving Company) each agree to (a) consult with each other before issuing any press release or otherwise making any public statement with respect to the transactions contemplated by this Agreement, (b) provide to the other Party for review a copy of any such press release or public statement and (c) not issue any such press release or make any such public statement prior to such consultation and review and the receipt of the prior written consent of the other Party (such consent not to be unreasonably withheld, conditioned or delayed), unless (and only to the extent) required by Applicable Law. Notwithstanding the foregoing, Avalon may disclose the subject matter of this Agreement to current and potential equityholders or investors.
5.10 Stockholders’ Meetings.
5.10.1 Avalon Annual Meeting. Subject to Article VII, Avalon shall take all action in accordance with the federal securities law, the DGCL, the applicable rules of Nasdaq, Avalon’s certificate of incorporation, as amended, and Avalon’s by-laws, as amended, necessary to convene the Avalon Annual Meeting to be held on the earliest practical date as reasonably determined by Avalon in light of the circumstances, and to obtain the consent and approval of Avalon’s stockholders with respect to the issuance of the Common Exchange Shares pursuant to the Acquisition, including (in the absence of conditions that would justify the termination of this Agreement) recommending such approval to Avalon’s stockholders.
5.10.2 Sen Lang Special Meeting. Subject to Article VII, the Sen Lang Parties shall take all action in accordance with the federal securities laws, BVI Law, Sen Lang’s certificate of incorporation, as amended, and Sen Lang’s by-laws, as amended, necessary to convene the Sen Lang Special Meeting to be held on the earliest practical date as reasonably determined by Avalon in light of the circumstances, and to obtain the consent and approval of the Sen Lang Shareholders with respect to this Agreement and the transactions contemplated hereby, including (in the absence of conditions that would justify the termination of this Agreement) recommending such approval to the Sen Lang Shareholders.
A-40
5.11 Preparation of the Proxy Statement.
5.11.1 Avalon and the Sen Lang Representative shall, as soon as is reasonably practicable, cooperate to prepare the Proxy Statement. Once both parties consent to the filing of the Proxy Statement with the SEC (which consent shall not be unreasonably withheld, delayed or conditioned), Avalon shall file the Proxy Statement with the SEC. If, at any time prior to the Closing, Avalon or the Sen Lang Representative shall obtain knowledge of any information contained in or omitted from the Proxy Statement that would require an amendment or supplement to the Proxy Statement, the party obtaining such knowledge will promptly so advise the other party in writing and both Sen Lang and Avalon will promptly take such action as shall be required to amend or supplement the Proxy Statement. Sen Lang shall promptly furnish to Avalon all financial and other information concerning it as may be required for the Proxy Statement and any supplements or amendments thereto. Avalon and the Sen Lang Representative shall cooperate in the preparation of the Proxy Statement in a timely fashion and shall use all reasonable efforts to clear the Proxy Statement with the Staff of the SEC. Each of Sen Lang and Avalon shall use all reasonable efforts to mail at the earliest practicable date to its stockholders the Proxy Statement, which shall include all information required under Applicable Law to be furnished to the Sen Lang Shareholders and Avalon’s stockholders in connection with the Acquisition and the transactions contemplated thereby. Avalon also shall take such other reasonable actions (other than qualifying to do business in any jurisdiction in which it is not so qualified or submitting to taxation in any jurisdiction in which it is not subject to taxation) required to be taken under any applicable state securities laws in connection with the issuance of Avalon Common Stock in the Acquisition. Notwithstanding any provision herein to the contrary, the Proxy Statement shall contain the audited consolidated financial statements described in clause “a” of Section 5.13.1.
5.11.2 Notwithstanding anything contained in this Agreement to the contrary, Avalon shall not be obligated to take any action under Section 5.11.1 unless and until the following conditions shall have been met: (i) Avalon shall have received the audited financial statements of the Acquired Companies and any other financial information of the Acquired Companies required for inclusion in the Proxy Statement and (ii) Avalon shall have received pro forma financial statements approved by Sen Lang and its auditors required to be included in the Proxy Statement, under SEC rules..
5.12 Nasdaq Listing. Avalon shall use its reasonable efforts to cause the Avalon Common Stock issuable pursuant to the Acquisition to be approved for listing on Nasdaq, subject to official notice of issuance, prior to the Closing.
5.13 Financial Statements for a Current Report on Form 8-K.
5.13.1 At the Closing, Sen Lang shall cause Friedman LLP to deliver to Avalon an executed consent, in form and substance reasonably satisfactory to Avalon and suitable for filing by Avalon with the SEC, which consent shall authorize Avalon to file with the SEC the report delivered pursuant to Section 5.13.1.
5.13.2 Upon Avalon’s request, contemporaneous with the delivery of the consolidated financial statements described in Section 5.13.1, Sen Lang shall cause Friedman LLP to make available to Avalon and its representatives the work papers generated in connection with such accounting firm’s audit of the audited consolidated financial statements delivered pursuant to Section 5.13.1.
A-41
5.13.3 Prior to the Closing, the Acquired Companies shall cooperate with Avalon in providing to Avalon such consolidated financial statements, financial data and accountants’ reports as Avalon shall reasonably request with respect to any filing that Avalon shall make under the Securities Act or the Exchange Act.
5.14 Business of the PRC Companies. Sen Lang and each of the Sen Lang Beneficial Shareholders shall use all reasonable efforts to take, or cause to be taken, all actions, and to do, or cause to be done, and to assist and cooperate with Avalon, in making sure that
(i) the business of each of the PRC Companies shall be restricted to its respective scope of business as stated in its business license and required for the carrying on of the Principal Business, and shall be carried out in accordance with the Control Documents;
(ii) each of the PRC Companies shall duly comply in all material respects with the laws applicable to such PRC Company in any jurisdiction in which such Group Company operates, is organized or licensed to do business;
(iii) each of the PRC Companies shall obtain, maintain and/or update all consents, approvals, licenses, permits or actions of, and make all filings with, in-charge government authorities or any other person needed for it to conduct its business;
(iv) each of the PRC Companies will maintain the eligibility for all the granted government finance subsidies that it is entitled to as of the Closing.
5.15 Control Documents. Sen Lang and each of the Sen Lang Beneficial Shareholders shall ensure that each party to the Control Documents fully performs its respective obligations under the Control Documents and use their best efforts to realize the business intention of the Control Documents. If the Control Documents become illegal, void or unenforceable under PRC laws after the date hereof, Sen Lang and each of the Sen Lang Beneficial Shareholders shall use their best efforts in good faith to devise a feasible alternative legal structure that gives effect as closely as possible the intentions of the parties in the Control Documents and the economic consequences thereto.
5.15.1.1 On and after the Closing, Avalon shall have the option to designate one or more corporate entities or individuals as nominee shareholder(s) (“Nominee Shareholder(s)”) of the OpCo. At Avalon’s request, the Sen Lang Beneficial Shareholders shall take all actions necessary or desirable to (i) issue and sell such equity interest as requested by Avalon for zero consideration, and (ii) enter into or revise the Control Documents in the agreed form, such that following such issuance and the entry into or revision of the Control Documents by the respective parties thereto, (A) Avalon or any of its respective Affiliates (as applicable) shall hold such percentage of equity interest in the OpCo that reflects Avalon’s control over the Acquired Companies, and (B) the PRC Subsidiary, shall continue to exercise control over the economic interest in, and the operations of, the OpCo, and the financial statements of the OpCo can be consolidated with those of the other Acquired Companies.
A-42
5.15.1.2 The Sen Lang Beneficial Shareholders agree to cooperate with Avalon with the restructuring of the Control Documents to reflect the Avalon’s ownership in the OpCo, which restructuring shall be carried out in a manner that will not result in any additional economic exposure for Avalon or its Affiliates.
5.15.1.3 In the case that the Applicable Laws allow a foreign entity to invest in the Principal Business, if so requested by Avalon, the Sen Lang Beneficial Shareholders shall use their best effects to transfer the Principal Business, together with the assets and properties related thereto, undertaken or owned by the OpCo to the PRC Subsidiary or another Subsidiary of Avalon as approved by Avalon, within a reasonable period of time designated by Avalon.
5.16 Circular 37. Each holder or beneficial owner of any equity securities of Sen Lang, including without limitations the Sen Lang Beneficial Shareholders, who is a “PRC Resident” as defined in Circular 37 and is subject to any of the registration or reporting requirements of Circular 37, shall take all necessary actions so as to comply with such reporting and/or registration requirements under Circular 37 within sixty (60) Business Days after the Closing, if they have not done so before the Closing.
5.17 Human Genetic Resources. If the competent authority challenges or prohibits the collection and use of human genetic resources by the Acquired Companies claiming that the Acquired Companies are controlled by a foreign investor, Sen Lang and each of the Sen Lang Beneficial Shareholders shall use their best efforts in good faith to devise a feasible alternative legal structure which gives effect as closely as possible the intentions of the parties and the economic consequences thereto.
5.18 PRC Tax Circular 7.
5.18.1 Each of the Parties hereby acknowledges, covenants and agrees that (i) Avalon shall have no obligation to pay any Tax in the PRC of any nature that is required by applicable PRC Tax Law to be paid by each Sen Lang Shareholder in its capacity of a Sen Lang Shareholder of the Sen Lang Shares arising in connection with the transactions contemplated hereby, and (ii) the Sen Lang Shareholders and Sen Lang Beneficial Shareholders agree to bear and pay any and all Tax of any nature that is required by applicable PRC Law to be paid by them arising in connection with the transactions contemplated hereby.
5.18.2 The Sen Lang Shareholders shall collectively engage and authorize a reputable PRC tax advisor (whose fees shall be borne by the Sen Lang Shareholders and not Avalon) to, and shall procure the PRC tax advisor to, file with the competent PRC Tax authorities all documents as required under the PRC State Taxation Administration Circular [2015] No. 7, as may be amended and supplemented, and other applicable PRC tax laws (the “PRC Tax Circular 7”) in connection with the transactions contemplated hereby within thirty (30) days after the date hereof, and shall (x) provide draft filings to Avalon for review within fifteen (15) days after the date hereof, consider in good faith any comments thereto by Avalon (any such comments shall be delivered to the Sen Lang Shareholders within ten (10) days after the receipt of the draft filings by Avalon, and permit Avalon to make a joint filing with the Sen Lang Shareholders (or to sign on the filing by the Sen Lang Shareholders) if Avalon so elects, and (y) provide Avalon with a copy of the official filing receipt issued by the competent PRC Tax authority evidencing that such Tax filings have been made in accordance with applicable Law as soon as reasonably practicable.
A-43
5.18.3 The Sen Lang Shareholders shall use their best efforts to coordinate with the competent PRC Tax authorities to complete the assessment regarding whether any Tax imposed pursuant to the PRC Tax Circular 7 in connection with the transactions contemplated hereby will be imposed on the Sen Lang Shareholders, and timely pay or cause to be timely paid such PRC Taxes due and payable in full (the “PRC Capital Gain Tax”) and obtain the tax clearance certificates therefor from the competent PRC Tax authorities (to the extent such certificates are issued) (the “Tax Clearance Certificates”).
5.18.4 The Sen Lang Shareholders covenant and agree to (A) keep Avalon informed of (i) any correspondence or communications with the competent PRC Tax authorities on these filings, (ii) the final assessment by the competent PRC Tax authorities of whether the PRC Capital Gain Tax will be imposed on the Sen Lang Shareholders, and (iii) if applicable, the final confirmation by the competent PRC Tax authorities of (x) the amount of the PRC Capital Gain Tax, and (y) the timing for payment of the PRC Capital Gain Tax, and (B) to provide Avalon with drafts and copies of any correspondence or filings in respect of the PRC Tax Circular 7 and a copy of the Tax Clearance Certificates within five (5) Business Days after obtaining the same from the competent PRC Tax authorities (to the extent such certificates are issued).
5.18.5 Notwithstanding anything in this Agreement to the contrary, (i) each Party shall cooperate with the other Party and each member of the Group Company as and to the extent reasonably requested by such other Party and such member of the Group Company in connection with the filing of any Tax returns or other tax filings or reports and in any threatened or actual proceeding with respect to Taxes, including the retention and (upon request) the provision of records, and (ii) nothing herein shall be deemed to prevent or restrict Avalon or such member of Avalon’s Group from making any Tax reporting or filing that is required or permitted to be made by Avalon or member of Avalon’s Group under applicable PRC Tax laws (including the PRC Tax Circular 7).
5.18.6 The Sen Lang Owners shall jointly and severally indemnify and hold harmless, on an after-Tax basis, Avalon forthwith on demand from and against all Taxes and all costs, expenses, demands, Liabilities, losses and damages incurred or suffered by Avalon arising or resulting from or in connection with any breach by the Sen Lang Shareholders or Sen Lang Beneficial Shareholders of any of their obligations under this Section 5.18 and the competent PRC Tax authorities not recognising in full Avalon’s actual cost of acquisition of the Sen Lang Shares as the cost basis of Avalon for the Sen Lang Shares in any future disposal of such Sen Lang Shares by Avalon.
5.19 Rule 144. The Sen Lang Owners understand and acknowledge that the Common Exchange Shares will be “restricted securities” as such term is used in Rule 144 under the Securities Act (“Rule 144”), will bear a restrictive legend restricting the transfer of such Common Exchange Shares in connection therewith for a period of six (6) months from the Closing Date and that the Common Exchange Shares will be indicated as restricted on Avalon’s shareholders’ register. The Sen Lang Owners covenant and agree to not transfer the Common Exchange Shares in violation of Rule 144. Following such six (6) month period, the Common Exchange Shares will be eligible for resale under Rule 144, subject to certain restrictions if such Person is an Affiliate of Avalon.
A-44
ARTICLE VI.
CONDITIONS
6.1 Conditions to the Obligations of Each Party. The obligations of Avalon, Sen Lang, the Sen Lang Shareholders, the Sen Lang Beneficial Shareholders, and the Sen Lang Representative to consummate the Acquisition and other transactions contemplated by this Agreement shall be subject to the satisfaction (or waiver by each Party, to the extent permitted by law) of the following conditions:
6.1.1 (i) This Agreement, the Acquisition and the other transactions contemplated hereby shall have been approved and adopted by the Sen Lang Shareholders in the manner required by Applicable Law, and (ii) the issuance of the Common Exchange Shares to be issued in the Acquisition shall have been approved by Avalon’s stockholders and Avalon in the manner required by Applicable Law and the applicable rules of Nasdaq.
6.1.2 No Governmental Authority of competent jurisdiction shall have enacted, issued, promulgated, enforced or entered any statute, rule, regulation, judgment, decree, injunction or other order which is in effect, which would prohibit consummation of the transactions contemplated by this Agreement or which would have an Avalon Material Adverse Effect after the Closing and after giving effect to consummation of the transactions contemplated by this Agreement.
6.1.3 The shares of Avalon Common Stock required to be issued pursuant to the Acquisition shall have been approved for listing on Nasdaq, subject to official notice of issuance.
6.2 Conditions to Avalon’s Obligations. The obligations of Avalon to consummate the Acquisition and the other transactions contemplated by this Agreement shall be subject to the fulfillment (or waiver by Avalon) prior to or at Closing of each of the following conditions:
6.2.1 The representations and warranties of the Acquired Companies set forth in ARTICLE III shall be true and correct in all material respects (other than representations and warranties which are qualified as to materiality, which representations and warranties shall be true in all respects) on the date hereof and on and as of the Closing Date as though made on and as of the Closing Date (except for representations and warranties made as of a specified date, which shall be measured only as of such specified date), except where the failure of such representations and warranties to be so true and correct (without giving effect to any limitations as to “materiality” or a Sen Lang Material Adverse Effect set forth therein) does not have, and is not reasonably likely to have, individually or in the aggregate, a Sen Lang Material Adverse Effect, provided that the representations and warranties set forth in Sections 3.1, 3.2, 3.3 and 3.13 shall be true and correct in all respects on the date hereof and on and as of the Closing Date as though made on and as of the Closing Date (except for representations and warranties made as of a specified date, which shall be measured as of such specified date).
A-45
6.2.2 the Acquired Companies shall have performed in all material respects each of its obligations under this Agreement and shall have complied in all material respects with each covenant to be performed and complied with by it under this Agreement at or prior to the Closing.
6.2.3 Since the date of this Agreement, there shall not have occurred any act, event or omission having or reasonably likely to have a Sen Lang Material Adverse Effect.
6.2.4 the Acquired Companies shall have obtained all authorizations, consents, waivers, approvals or other actions described in Section 6.2.4 of the Sen Lang Disclosure Schedule required in connection with the execution, delivery and performance of this Agreement by the Acquired Companies (the “Sen Lang Approvals”) and the Sen Lang Approvals shall be in full force and effect as of the Closing Date. Avalon shall have obtained all authorizations, consents, waivers, approvals or other actions described in Section 6.2.4 of the Avalon Disclosure Schedule (the “Avalon Approvals”) and the Avalon Approvals shall be in full force and effect as of the Closing Date.
6.2.5 There shall not be pending any Action which (a) in the reasonable judgment of Avalon’s Board of Directors, is reasonably likely to cause an Avalon Material Adverse Effect after the Closing giving effect to consummation of the transactions contemplated by this Agreement and (b) either (i) challenges or seeks to restrain or prohibit the consummation of the Acquisition or any of the other transactions contemplated by this Agreement, (ii) seeks to prohibit or limit the ownership or operation by Avalon, Sen Lang or any of their respective subsidiaries of, or to compel Avalon, Sen Lang or any of their respective subsidiaries to dispose of or hold separate, any material portion of the business or assets of Avalon, Sen Lang or any of their respective subsidiaries, as a result of the Acquisition or any of the other transactions contemplated by this Agreement, (iii) seeks to impose limitations on the ability of Avalon to acquire or hold, or exercise full rights of ownership of, any shares of capital stock of the Surviving Company, including the right to vote such capital stock of the Surviving Company on all matters properly presented to the stockholders of the Surviving Company, or (iv) seeks to prohibit Avalon or any subsidiary of Avalon from effectively controlling in any material respect the business or operations of Avalon or the subsidiaries of Avalon including the Surviving Company.
6.2.6 Prior to or at the Closing, Sen Lang shall have delivered to Avalon the following:
6.2.7.1 a certificate of the chief executive officer (or comparable officer) of Sen Lang (executed on behalf of the Acquired Companies), dated as of the Closing Date, to the effect that (1) the Person signing such certificate is familiar with this Agreement and (2) to such Person’s knowledge, the conditions specified in Sections 6.2.1, 6.2.2 and 6.2.3 have been satisfied;
6.2.7.2 a certificate of the Secretary or Assistant Secretary (or comparable officer) of Sen Lang (executed on behalf of the Acquired Companies), dated as of the Closing Date, as to the incumbency of any officer of such entity executing this Agreement or any document related hereto; and
A-46
6.2.7.3 a copy of (1) the certificate of incorporation, as amended, of each Acquired Company, certified such Acquired Company’s jurisdiction of incorporation and dated not earlier than fifteen (15) days prior to the Closing Date, (2) a good standing certificate (or jurisdictional equivalent thereof) for each Acquired Company from each of its respective jurisdictions of operations, dated not earlier than fifteen (15) days prior to the Closing Date and confirming that such Acquired Company is in good standing in such jurisdiction, (3) the bylaws, as amended, of each Acquired Company, certified by the Secretary or Assistant Secretary (or comparable officer) of Sen Lang (on behalf of each Acquired Company) as of the Closing Date, and (4) the resolutions of Sen Lang’s Board of Directors (on behalf of the Acquired Companies) authorizing the execution, delivery and consummation of this Agreement and the transactions contemplated hereby, certified by the Secretary or Assistant Secretary (or comparable officer) of Sen Lang as of the Closing Date.
6.2.7 All reports of Sen Lang’s independent accountants relating to the Acquired Companies’ audited consolidated financial statements filed with (or incorporated by reference in any document filed with) the SEC subsequent to the date hereof and prior to the Closing shall certify, without qualification or exception, that such financial statements (a) have been prepared in accordance with generally accepted accounting principles consistently applied during the periods involved and (b) fairly present, in all material respects, the consolidated financial position of the entities described therein as of the dates thereof and the consolidated results of operations and consolidated cash flows of such entities for the periods presented.
6.2.8 The requisite vote (under Applicable Law and the rules and regulations of Nasdaq) of Avalon’s stockholders to authorize the issuance of Avalon Common Stock hereunder shall have been obtained.
6.2.9 The Avalon Equity Financing shall have been consummated no later than concurrently with the Closing.
6.2.10 Avalon shall have received a copy of the Escrow Agreement, duly executed by the Sen Lang Representative and the Escrow Agent.
6.2.11 Avalon shall have received an AI Letter for each Sen Lang Shareholder, duly executed by such Sen Lang Shareholder and its respective Sen Lang Beneficial Shareholder, and each AI Letter shall be true, accurate and complete as of the Closing.
6.2.12 The Control Documents shall have been duly executed and delivered by the relevant parties, to the satisfaction of Avalon. The Sen Lang Beneficial Shareholders have, in accordance with the Control Documents, pledged all the shares of OpCo they hold to the PRC Subsidiary and have completed the share pledge registration with the competent company registry, and have provided the relevant notice of registration of share pledge (股权出质登记通知书 in Chinese) issued by such company registry to Avalon.
6.2.13 Except for North United Investment Ltd. and DBVest Limited, each holder or beneficial owner of any equity securities of Sen Lang, including without limitations the Sen Lang Beneficial Shareholders, who is a “PRC Resident” as defined in Circular 37 and is subject to any of the registration or reporting requirements of Circular 37, shall have taken all necessary actions so as to comply with such reporting and/or registration requirements under Circular 37; provided, that, if such reporting and/or registration requirements have not been completed as of Closing, and Avalon elects, in its sole and absolute discretion, to waive this condition, such reporting and/or registration shall have been completed within sixty (60) Business Days after the Closing.
A-47
6.2.14 OpCo shall have completed the sale of 100% of the equity interests in Hebei Senlang Taihe Biotechnology Co., Ltd (河北森朗泰禾生物科技有限公司 in Chinese) to Avalon’s satisfaction (including, without limitation, satisfaction as to the sale price) and OpCo shall have provided the relevant notice of registration in connection with such sale issued by the competent company registry to Avalon.
6.2.15 OpCo shall have registered its lab located at the Runjiang Headquarter International Park as a branch company and shall have provided the relevant notice of the establishment of the branch company issued by the competent company registry to Avalon.
6.3 Conditions to Sen Lang’s Obligations. The obligations of the Sen Lang Parties to consummate the transactions contemplated by this Agreement shall be subject to the fulfillment (or waiver by the Sen Lang Representative) at or prior to the Closing of each of the following conditions:
6.3.1 The representations and warranties of Avalon set forth in ARTICLE IV shall be true and correct in all material respects (other than representations and warranties which are qualified as to materiality, which representations and warranties shall be true in all respects) on the date hereof and on and as of the Closing Date as though made on and as of the Closing Date (except for representations and warranties made as of a specified date, which shall be measured only as of such specified date), except where the failure of such representations and warranties to be so true and correct (without giving effect to any limitations as to “materiality” or an Avalon Material Adverse Effect set forth therein) does not have, and is not reasonably likely to have, individually or in the aggregate, an Avalon Material Adverse Effect, provided that the representations and warranties set forth in Sections 4.1, 4.2 and 4.19 shall be true and correct in all respects on the date hereof and on and as of the Closing Date as though made on and as of the Closing Date (except for representations and warranties made as of a specified date, which shall be measured as of such specified date).
6.3.2 Avalon shall have performed in all material respects each of its obligations under this Agreement and shall have complied in all material respects with each covenant to be performed and complied with by Avalon this Agreement at or prior to the Closing.
6.3.3 Avalon shall have obtained all of the Avalon Approvals and the Avalon Approvals shall be in full force and effect as of the Closing Date.
6.3.4 Prior to or at the Closing, Avalon shall have delivered to Sen Lang the following:
6.3.4.1 a certificate of the chief executive officer (or comparable officer) of Avalon, dated as of the Closing Date, to the effect that (1) the Person signing such certificate is familiar with this Agreement and (2) to such Person’s knowledge, the conditions specified in Sections 6.3.1, 6.3.2 and 6.3.5 have been satisfied;
A-48
6.3.4.2 a certificate of the Secretary or Assistant Secretary (or comparable officer) of Avalon, dated as of the Closing Date, as to the incumbency of any officer of Avalon executing this Agreement or any document related hereto; and
6.3.4.3 a copy of (1) the certificate of incorporation, as amended, of Avalon, certified by the Delaware Secretary of State and dated not earlier than fifteen (15) days prior to the Closing Date, (2) a certificate of the Delaware Secretary of State, dated not earlier than fifteen (15) days prior to the Closing Date and confirming that Avalon is in good standing in the State of Delaware, (3) the bylaws, as amended, of Avalon, certified by the Secretary or Assistant Secretary (or comparable officer) of Avalon as of the Closing Date, and (4) the resolutions of Avalon’s Board of Directors (or Committee thereof) authorizing the execution, delivery and consummation of this Agreement and the transactions contemplated hereby, certified by the Secretary or Assistant Secretary (or comparable officer) of Avalon as of the Closing Date.
6.3.5 Since the date of this Agreement, there shall not have occurred any act, event or omission having or reasonably likely to have an Avalon Material Adverse Effect.
6.3.6 The Sen Lang Representative shall have received a copy of the Escrow Agreement, duly executed by Avalon and the Escrow Agent
6.3.7 All reports of Avalon’s independent accountants relating to Avalon’s audited consolidated financial statements filed with (or incorporated by reference in any document filed with) the SEC subsequent to the date hereof and prior to the Closing shall certify, without qualification or exception, that such financial statements (a) have been prepared in accordance with generally accepted accounting principles consistently applied during the periods involved and (b) fairly present, in all material respects, the consolidated financial position of the entities described therein as of the dates thereof and the consolidated results of operations and consolidated cash flows of such entities for the periods presented.
ARTICLE VII.
TERMINATION
7.1 Termination. This Agreement may be terminated and the Acquisition may be abandoned at any time prior to the Closing (notwithstanding any approval of this Agreement by the Sen Lang Shareholders and/or Avalon’s stockholders):
7.1.1 by mutual written consent of Avalon and the Sen Lang Representative;
7.1.2 by either Avalon or the Sen Lang Representative if there shall be any law or regulation that, as supported by the written opinion of outside legal counsel, makes consummation of the Acquisition illegal or otherwise prohibited, or if any judgment, injunction, order or decree of a court or other competent Governmental Authority enjoining Avalon or the Sen Lang Representative from consummating the Acquisition shall have been entered and such judgment, injunction, order or decree shall have become final and nonappealable;
A-49
7.1.3 by either Avalon or the Sen Lang Representative if the Acquisition shall not have been consummated before the Outside Date (as hereinafter defined), provided, however, that the right to terminate this Agreement under this Section 7.1.3 shall not be available to any party whose failure or whose affiliate’s failure to perform any material covenant or obligation under this Agreement has been the cause of or resulted in the failure of the Acquisition to occur on or before such date;
7.1.4 by Avalon if the Board of Directors of Sen Lang shall withdraw, modify or change the Sen Lang Board Recommendation in a manner adverse to Avalon or by the Sen Lang Representative if the Board of Directors of Avalon shall withdraw, modify or change the Avalon Board Recommendation in a manner adverse to the Sen Lang Owners;
7.1.5 by either Avalon or the Sen Lang Representative if at the Sen Lang Special Meeting (including any adjournment or postponement thereof) the requisite vote (under Applicable Law) of the Sen Lang Shareholders to approve the Acquisition and the transactions contemplated hereby shall not have been obtained;
7.1.6 by either Avalon or the Sen Lang Representative if at the Avalon Annual Meeting (including any adjournment or postponement thereof) the requisite vote (under Applicable Law and the rules and regulations of Nasdaq) of Avalon’s stockholders to authorize the issuance of Avalon Common Stock in the Acquisition shall not have been obtained;
7.1.7 by either Avalon or the Sen Lang Representative if any representation or warranty made in this Agreement (including the Sen Lang Disclosure Schedule and the Avalon Disclosure Schedule) for its benefit is untrue in any material respect (other than representations and warranties which are qualified as to materiality, which representations and warranties will give rise to termination if untrue in any respect); provided that, in each case, (a) the party seeking to terminate this Agreement is not then in material breach of any material representation or warranty contained in this Agreement, (b) such untrue representation or warranty cannot be or has not been cured within thirty (30) days after receipt of written notice of such breach and (c) in the case of Sen Lang, except for the representations and warranties contained in Sections 3.1, 3.2, 3.3 and 3.13, and in the case of Avalon, except for the representations and warranties contained in Sections 4.1, 4.2 and 4.19, such untrue representation and warranty has, or is reasonably likely to have, a Sen Lang Material Adverse Effect or an Avalon Material Adverse Effect, as the case may be and in each case after the Closing and after giving effect to consummation of the transactions contemplated by this Agreement;
7.1.8 by either Avalon or the Sen Lang Representative if the other party shall have defaulted in the performance of any material covenant or agreement under this Agreement; provided that, in each case, (a) the Party seeking to terminate this Agreement has complied with its covenants and agreements under this Agreement in all material respects and (b) such failure to comply cannot be or has not been cured within thirty (30) days after receipt of written notice of such default;
A-50
7.1.9 by Avalon if any authorization, consent, waiver or approval required for the consummation of the transactions contemplated hereby shall require the divestiture or cessation of any of the present business or operations conducted by Avalon or its Subsidiaries or any Acquired Company or shall impose any other material condition or requirement, which divestiture, cessation, condition or requirement, in the reasonable judgment of Avalon’s Board of Directors, would be reasonably likely to have an Avalon Material Adverse Effect after the Closing giving effect to consummation of the transactions contemplated by this Agreement;
7.1.10 by Avalon, in the event that the conditions to its obligations set forth in ARTICLE VI have not been satisfied or waived by the date set for the Closing or in the event that such conditions cannot possibly be satisfied prior to the Outside Date, provided that Avalon is not then in material breach of any material representation, warranty, covenant or other agreement contained in this Agreement; or
7.1.11 by Sen Lang, in the event that the conditions to its obligations set forth in ARTICLE VI have not been satisfied or waived by the date set for the Closing or in the event that such conditions cannot possibly be satisfied prior to the Outside Date, provided that Sen Lang is not then in material breach of any material representation, warranty, covenant or other agreement contained in this Agreement.
For purposes of this Agreement, the “Outside Date” means December 31, 2021.
7.2 Effect of Termination. In the event of the termination of this Agreement pursuant to Section 7.1, this Agreement, except for any provisions relating to the confidentiality obligations of the parties hereto to each other and the provisions of this Section 7.2 and Section 9.12, shall become void and have no effect, without any liability on the part of any party or its directors, officers or stockholders. Notwithstanding the foregoing, nothing in this Section 7.2 shall relieve any party to this Agreement of liability for a material breach of any material provision of this Agreement.
ARTICLE VIII.
SURVIVAL AND INDEMNIFICATION
8.1 Survival of Representations and Warranties.
8.1.1 All representations and warranties of the Sen Lang Parties and Acquired Companies contained in this Agreement (including all schedules and exhibits hereto and all certificates, documents, instruments and undertakings furnished pursuant to this Agreement) shall survive the Closing through and until and including the twenty-four (24) month anniversary of the Closing Date (the “Expiration Date”), other than representations and warranties of the Sen Lang Parties and Acquired Companies contained in this Agreement with respect to Taxes and Indebetdness, which shall survive the Closing through and until sixty (60) days after the expiration of the applicable statute of limitations for the underlying item; provided, however, that any claim based in whole or in part upon fraud, willful misconduct or intentional misrepresentation (collectively, “Fraud Claims”) shall survive indefinitely. If a Claim Notice (as defined herein) for a claim of a breach of any representation or warranty has been given before the Expiration Date, then the relevant representations and warranties shall survive as to such claim, until the claim has been finally resolved. All covenants, obligations and agreements of the Sen Lang Parties and the Acquired Companies contained in this Agreement (including the Sen Lang Disclosure Schedule, all schedules and exhibits hereto and all certificates, documents, instruments and undertakings furnished by any Sen Lang Party or Acquired Companypursuant to this Agreement), including any indemnification obligations, shall survive the Closing and continue until fully performed in accordance with their terms.
A-51
8.1.2 The representations and warranties of Avalon contained in this Agreement or in any certificate or instrument delivered by or on behalf of Avalon pursuant to this Agreement shall not survive the Closing, and from and after the Closing, Avalon, its Affiliates, and its and their respective managers, directors, officers, employees, independent contractors, consultants, advisors (including financial advisors, counsel and accountants), agents and other legal representatives shall not have any further obligations, nor shall any claim be asserted or Action be brought against Avalon or its managers, directors, officers, employees, independent contractors, consultants, advisors (including financial advisors, counsel and accountants), agents and other legal representatives with respect thereto. The covenants and agreements made by Avalon in this Agreement or in any certificate or instrument delivered pursuant to this Agreement, including any rights arising out of any breach of such covenants or agreements, shall not survive the Closing, except for those covenants and agreements contained herein and therein that by their terms apply or are to be performed in whole or in part after the Closing (which such covenants shall survive the Closing and continue until fully performed in accordance with their terms).
8.1.3 Indemnification by Sen Lang Owners. Subject to the terms and conditions of this ARTICLE VIII and as acknowledged in the AI Letter executed by each Sen Lang Owner, from and after the Closing, the Sen Lang Owners and their respective successors and assigns (each, with respect to any claim made pursuant to this Agreement, an “Indemnifying Party”) will severally and jointly indemnify, defend and hold harmless Avalon, its Affiliates and each of its and their respective officers, directors, managers, employees, successors and permitted assigns (each, with respect to any claim made pursuant to this Agreement, an “Indemnified Party”) from and against any and all losses, actions, orders, liabilities, damages (including consequential damages), diminution in value, Taxes, interest, penalties, Encumbrances, amounts paid in settlement, costs and expenses (including reasonable expenses of investigation and court costs and reasonable attorneys’ fees and expenses), (any of the foregoing, a “Loss”) paid, suffered or incurred by, or imposed upon, any Indemnified Party to the extent arising in whole or in part out of or resulting directly or indirectly from (whether or not involving a Third Party Claim): (a) the breach of any representation or warranty made by any Sen Lang Party or Acquired Company set forth in this Agreement or in any Ancillary Document or certificate delivered by any Sen Lang Party or Acquired Company; (b) the breach of any covenant or agreement on the part of any Sen Lang Party or Acquired Company set forth in this Agreement or in any Ancillary Document or certificate delivered by any Sen Lang Party or Acquired Company; (c) any Action by Person(s) who were holders of equity securities of an Acquired Company, including options, warrants, convertible debt or other convertible securities or other rights to acquire equity securities of an Acquired Company, prior to the Closing arising out of the sale, purchase, termination, cancellation, expiration, redemption or conversion of any such securities; (d) any debts, liabilities and obligations of the Acquired Companies existing or arising prior to the Closing or from and after the Closing due to events occurring prior to the Closing; (e) the Control Documents becoming illegal, void, unenforceable or ineffective, as determined by Avalon in its sole and absolute discretion, under Applicable Law; or (f) a competent authority challenging or prohibiting the collection and use of human genetic resources by the Acquired Companies.
A-52
8.2 Limitations and General Indemnification Provisions.
8.2.1 The maximum aggregate amount of indemnification payments to which the Indemnifying Parties will be obligated to pay in the aggregate (excluding Fraud Claims and Control Claims (as defined herein)) shall not exceed the amount of the Escrow Property in the Escrow Account at such time, and in the case of Fraud Claims and Control Claims, shall not exceed an amount equal to the Common Exchange Shares actually paid by Avalon to the Sen Lang Shareholders.
8.2.2 In no event shall any Indemnified Party be entitled to recover or make a claim for any amounts in respect of, and in no event shall Losses be deemed to include any punitive, special or exemplary damages except to the extent actually paid to a third party in a Third Party Claim.
8.2.3 For purposes of determining whether there has been a breach giving rise to the indemnification claim, and determining the amount of Losses under this ARTICLE VIII, all of the representations, warranties and covenants set forth in this Agreement (including the Sen Lang Disclosures hereto) or any Ancillary Document that are qualified by materiality, Material Adverse Effect or words of similar import or effect will be deemed to have been made without any such qualification.
8.2.4 No investigation or knowledge by an Indemnified Party or its Representatives of a breach of a representation, warranty, covenant or agreement of an Indemnifying Party shall affect the representations, warranties, covenants and agreements of the Indemnifying Party or the recourse available to the Indemnified Parties under any provision of this Agreement, including this ARTICLE VIII, with respect thereto.
8.2.5 The amount of any Losses suffered or incurred by any Indemnified Party shall be reduced by the amount of any insurance proceeds paid to the Indemnified Party or any Affiliate thereof as a reimbursement with respect to such Losses (and no right of subrogation shall accrue to any insurer hereunder, except to the extent that such waiver of subrogation would prejudice any applicable insurance coverage), net of the costs of collection and the increases in insurance premiums resulting from such Loss or insurance payment.
8.3 Indemnification Procedures.
8.3.1 Avalon shall have the sole right to act on behalf of the Indemnified Parties with respect to any indemnification claims made pursuant to this ARTICLE VIII, including bringing and settling any indemnification claims hereunder and receiving any notices on behalf of the Indemnified Parties. The Sen Lang Representative shall have the sole right to act on behalf of the Indemnifying Parties with respect to any indemnification claims made pursuant to this ARTICLE VIII, including defending and settling any indemnification claims hereunder and receiving any notices on behalf of the Indemnifying Parties.
A-53
8.3.2 In order to make a claim for indemnification hereunder, Avalon on behalf of an Indemnified Party must provide written notice (a “Claim Notice”) of such claim to the Sen Lang Representative on behalf of the Indemnifying Parties and to the Escrow Agent, which Claim Notice shall include (i) a reasonable description of the facts and circumstances which relate to the subject matter of such indemnification claim to the extent then known and (ii) the amount of Losses suffered by the Indemnified Party in connection with the claim to the extent known or reasonably estimable (provided, that Avalon may thereafter in good faith adjust the amount of Losses with respect to the claim by providing a revised Claim Notice to the Sen Lang Representative and the Escrow Agent); provided, that the copy of any Claim Notice provided to the Escrow Agent shall be redacted for any confidential or proprietary information of the Indemnifying Party or the Indemnified Party described in clause (i).
8.3.3 In the case of any claim for indemnification under this ARTICLE VIII arising from a claim of a third party (including any Governmental Authority) (a “Third Party Claim”), Avalon must give a Claim Notice with respect to such Third Party Claim to the Sen Lang Representative promptly (but in no event later than thirty (30) days) after the Indemnified Party’s receipt of notice of such Third Party Claim; provided, that the failure to give such notice will not relieve the Indemnifying Party of its indemnification obligations except to the extent that the defense of such Third Party Claim is materially and irrevocably prejudiced by the failure to give such notice. The Sen Lang Representative will have the right to defend and to direct the defense against any such Third Party Claim in its name and at its expense, and with counsel selected by the Sen Lang Representative, unless (i) the Sen Lang Representative fails to acknowledge fully to Avalon the obligations of the Indemnifying Party to the Indemnified Party within twenty (20) days after receiving notice of such Third Party Claim or contests, in whole or in part, its indemnification obligations therefor or (ii) at any time while such Third Party Claim is pending, (A) there is a conflict of interest between the Sen Lang Representative on behalf of the Indemnifying Party and Avalon on behalf of the Indemnified Party in the conduct of such defense, (B) the applicable third party alleges a Fraud Claim or Control Claim, (C) such claim is criminal in nature, could reasonably be expected to lead to criminal Actions, or seeks an injunction or other equitable relief against the Indemnified Party, or (D) the amount of the Third Party Claim exceeds or is reasonably expected to exceed the value of the remaining Escrow Property in the Escrow Account (after deducting any amounts for pending but unresolved indemnification claims and resolved but unpaid indemnification claims). If the Sen Lang Representative on behalf of the Indemnifying Party elects, and is entitled, to compromise or defend such Third Party Claim, it will within twenty (20) days (or sooner, if the nature of the Third Party Claim so requires) notify Avalon of its intent to do so, and Avalon and the Indemnified Party will, at the request and expense of the Sen Lang Representative, cooperate in the defense of such Third Party Claim. If the Sen Lang Representative on behalf of the Indemnifying Party elects not to, or at any time is not entitled under this Section 8.3.3 to, compromise or defend such Third Party Claim, fails to notify Avalon of its election as herein provided or refuses to acknowledge or contests its obligation to indemnify under this Agreement, Avalon on behalf of the Indemnified Party may pay, compromise or defend such Third Party Claim. Notwithstanding anything to the contrary contained herein, the Indemnifying Party will have no indemnification obligations with respect to any such Third Party Claim which is settled by the Indemnified Party or Avalon without the prior written consent of the Sen Lang Representative on behalf of the Indemnifying Party (which consent will not be unreasonably withheld, delayed or conditioned); provided, however, that notwithstanding the foregoing, the Indemnified Party will not be required to refrain from paying any Third Party Claim which has matured by a final, non-appealable order of a Governmental Authority, nor will it be required to refrain from paying any Third Party Claim where the delay in paying such claim would result in the foreclosure of a Encumbrance upon any of the property or assets then held by the Indemnified Party or where any delay in payment would cause the Indemnified Party material economic loss. The Sen Lang Representative’s right on behalf of the Indemnifying Party to direct the defense will include the right to compromise or enter into an agreement settling any Third Party Claim; provided, that no such compromise or settlement will obligate the Indemnified Party to agree to any settlement that that requires the taking or restriction of any action (including the payment of money and competition restrictions) by the Indemnified Party other than the execution of a release for such Third Party Claim and/or agreeing to be subject to customary confidentiality obligations in connection therewith, except with the prior written consent of Avalon on behalf of the Indemnified Party (which consent will not be unreasonably withheld, delayed or conditioned). Notwithstanding the Sen Lang Representative’s right on behalf of the Indemnifying Party to compromise or settle in accordance with the immediately preceding sentence, the Sen Lang Representative on behalf of the Indemnifying Party may not settle or compromise any Third Party Claim over the objection of Avalon on behalf of the Indemnified Party; provided, however, that consent by Avalon on behalf of the Indemnified Party to settlement or compromise will not be unreasonably withheld, delayed or conditioned. Avalon, on behalf of the Indemnified Party, will have the right to participate in the defense of any Third Party Claim with counsel selected by it subject to the Sen Lang Representative’s right on behalf of the Indemnifying Party to direct the defense.
A-54
8.3.4 With respect to any direct indemnification claim that is not a Third Party Claim, the Sen Lang Representative, on behalf of the Indemnifying Party, will have a period of thirty (30) days after receipt of the Claim Notice to respond thereto. If the Sen Lang Representative, on behalf of the Indemnifying Party, does not respond within such thirty (30) days, the Sen Lang Representative, on behalf of the Indemnifying Party, will be deemed to have accepted responsibility for the Losses set forth in such Claim Notice subject to the limitations on indemnification set forth in this ARTICLE VIII and will have no further right to contest the validity of such Claim Notice. If the Sen Lang Representative responds within such thirty (30) days and rejects such claim in whole or in part, Avalon on behalf of the Indemnified Party will be free to pursue such remedies as may be available under this Agreement, any Ancillary Documents or Applicable Law.
8.4 Indemnification Payments. Any indemnification claims against the Indemnifying Parties (other than for Fraud Claims or Control Claims) shall be satisfied solely by the Escrow Property (with such indemnification first be applied against the Escrow Shares and then against any other Escrow Property), and no Indemnifying Party shall be required to make any out-of-pocket payment for indemnification other than in connection with Fraud Claims or Control Claims. Any indemnification obligation of an Indemnifying Party under this ARTICLE VIII will be paid within five (5) Business Days after the determination of such obligation in accordance with this ARTICLE VIII (and Avalon and the Sen Lang Representative will provide or cause to be provided to the Escrow Agent any written instructions or other information or documents required by the Escrow Agent to do so). Notwithstanding anything to the contrary contained herein, any indemnification payments will be made to Avalon or its successors. With respect to any indemnification payment, the value of each Escrow Share or any other share of Avalon Common Stock for purposes of determining the indemnification payment shall be the Avalon Per Share Price. Any Escrow Shares or other shares of Avalon Common Stock received by Avalon as an indemnification payment shall be promptly cancelled by Avalon after its receipt thereof. Without limiting any of the foregoing or any other rights of the Indemnified Parties under this Agreement or any Ancillary Document or at law or equity, in the event that an Indemnifying Party fails or refuses to promptly indemnify an Indemnified Party as provided herein or otherwise fails or refuses to make any payments required under any Ancillary Document, in either case, where it is established that such Indemnifying Party is obligated to provide such indemnification or to make such payment, the applicable Indemnified Party shall, in its sole discretion, be entitled to claim a portion of the shares of Avalon Common Stock then owned by such Indemnifying Party up to an amount equal in value (based on the Avalon Per Share Price) to the amount owed by such Indemnifying Party. In the event that such Indemnifying Party fails to promptly transfer any such shares of Avalon Common Stock pursuant to this Section 8.4, Avalon shall be and hereby is authorized as the attorney-in-fact for such Indemnifying Party to transfer such shares of Avalon Common Stock to the proper recipient thereof as required by this Section 8.4, and may transfer such shares of Avalon Common Stock and cancel the stock certificates for such shares on the books and records of Avalon and issue new stock certificates to such transferee and may instruct its agents and any exchanges on which Avalon Common Stock is listed or traded to do the same.
8.5 Exclusive Remedy. From and after the Closing, except with respect to Fraud Claims, Control Claims or claims seeking injunctions, specific performance or other equitable relief (including pursuant to Section 9.10), or claims under the terms of the Letters of Transmittal or other Ancillary Documents, indemnification pursuant to this ARTICLE VIII shall be the sole and exclusive remedy for the Parties with respect to matters arising under this Agreement of any kind or nature, including for any misrepresentation or breach of any warranty, covenant, or other provision contained in this Agreement or in any certificate or instrument delivered pursuant to this Agreement or otherwise relating to the subject matter of this Agreement, including the negotiation and discussion thereof.
A-55
ARTICLE IX.
MISCELLANEOUS
9.1 Amendments, Extensions and Waivers. No amendment of any provision of this Agreement shall be valid unless the same shall be in writing and signed by Avalon and the Sen Lang Representative. Subject to the limitations set forth herein, at any time prior to the Closing, the Parties may extend the time for the performance of any of the obligations or other acts of the other Parties. No waiver by any Party of any provision of this Agreement or any default, misrepresentation, or breach of warranty or covenant hereunder, whether intentional or not, shall be valid unless the same shall be in writing and signed by the Party making such waiver nor shall such waiver be deemed to extend to any prior or subsequent default, misrepresentation, or breach of warranty or covenant hereunder or affect in any way any rights arising by virtue of any prior or subsequent such occurrence.
9.2 Notices. All notices, requests, demands, claims, and other communications hereunder shall be in writing. Any notice, request, demand, claim, or other communication hereunder shall be deemed duly given (i) when delivered personally to the recipient, (ii) one (1) Business Day after being sent to the recipient by reputable overnight courier service (charges prepaid), (iii) one (1) Business Day after being sent to the recipient by facsimile transmission or electronic mail, or (iv) four (4) Business Days after being mailed to the recipient by certified or registered mail, return receipt requested and postage prepaid, and addressed to the intended recipient as set forth below:
9.2.1 if to Avalon:
Avalon GloboCare Corp.
4400 Route 9 South
Suite 3100
Freehold, New Jersey 07728
Attention: Meng Li, Chief Operating Officer
E-mail: meng@avalon-globocare.com
with a copy (which shall not constitute notice) to:
Lowenstein Sandler LLP
One Lowenstein Drive
Roseland, New Jersey 07068
Attention: Steven M. Skolnick, Esq.
E-mail: sskolnick@lowenstein.com
9.2.2 if to the Sen Lang Representative:
Ding Wei
B09,5/F, Reignwood Center, Jianguomenwai Ave,
Beijing, China
E-mail: Dingwei@senlangbio.com
with a copy (which shall not constitute notice) to:
Co-Effort LLP
35th Floor, Huaneng United Building, 958 Lujiazui Ring Road,
Shanghai, China
Attention: Jin Yuan
E-mail: Jinyuan@co-effort.com
Any Party may change the address to which notices, requests, demands, claims, and other communications hereunder are to be delivered by giving the other Parties notice in the manner herein set forth.
A-56
9.3 Interpretation; Construction.
9.3.1 When a reference is made in this Agreement to an Article or Section, such reference shall be to an Article or Section of this Agreement unless otherwise indicated. The headings and the table of contents contained in this Agreement are for reference purposes only and shall not affect in any way the meaning or interpretation of this Agreement.
9.3.2 The Parties have participated jointly in the negotiation and drafting of this Agreement. In the event an ambiguity or question of intent or interpretation arises, this Agreement shall be construed as if drafted jointly by the Parties and no presumption or burden of proof shall arise favoring or disfavoring any Party by virtue of the authorship of any of the provisions of this Agreement. Any reference to any federal, state, local, or non-U.S. statute or law shall be deemed also to refer to all rules and regulations promulgated thereunder, unless the context requires otherwise. Unless otherwise indicated to the contrary herein by the context or use thereof: (i) the words, “herein,” “hereto,” “hereof” and words of similar import refer to this Agreement as a whole, including the Disclosure Schedules and exhibits, and not to any particular section, subsection, paragraph, subparagraph or clause contained in this Agreement; (ii) masculine gender shall also include the feminine and neutral genders, and vice versa; (iii) words importing the singular shall also include the plural, and vice versa; and (iv) the words “include,” “includes” or “including” shall be deemed to be followed by the words “without limitation”.
9.3.3 The Exhibits, Sen Lang Disclosure Schedule, Avalon Disclosure Schedule and other schedules identified in this Agreement are incorporated herein by reference and made a part hereof.
9.4 “Knowledge” Defined. For purposes of this Agreement, “knowledge” of a Person means the actual knowledge of such Person, or all officers of such Person with a title of executive vice president or higher, after reasonable inquiry.
9.5 Counterparts. This Agreement may be executed in one or more counterparts, each of which shall be deemed an original but all of which together will constitute one and the same instrument. Delivery of an executed counterpart of a signature page to this Agreement by facsimile or scanned pages shall be effective as delivery of a manually executed counterpart to this Agreement.
9.6 Entire Agreement. This Agreement, the Ancillary Documents and the Letter Agreement, dated August 27, 2020, from Avalon and accepted and agreed as of August 29, 2020 by Hebei Senlangbio Technology Co. Ltd. constitute the entire agreement among the parties and supersede all prior agreements and understandings, agreements or representations by or among the parties, written and oral, with respect to the subject matter hereof and thereof.
9.7 Third-Party Beneficiaries. Nothing in this Agreement, express or implied, is intended or shall be construed to confer any rights or remedies upon any Person other than the Parties.
9.8 Governing Law. Except to the extent that the laws of the jurisdiction of organization of any party hereto, or any other jurisdiction, are mandatorily applicable to the Acquisition or to matters arising under or in connection with this Agreement, this Agreement shall be governed by the laws of the State of Delaware. All Actions arising out of or relating to this Agreement shall be heard and determined exclusively in any state or federal court sitting in the State of Delaware.
A-57
9.9 Consent to Jurisdiction; Venue; Waiver of Jury Trial.
9.9.1 Each of the parties hereto irrevocably submits to the exclusive jurisdiction of the state courts of Delaware and the United States District Court for the District of Delaware, for the purpose of any Action arising out of or relating to this Agreement and each of the parties hereto irrevocably agrees that all claims in respect to such Action shall be heard and determined exclusively in any Delaware state or federal court. Each of the parties hereto agrees that a final judgment in any Action shall be conclusive and may be enforced in other jurisdictions by suit on the judgment or in any other manner provided by law.
9.9.2 Each of the parties hereto irrevocably consents to the service of any summons and complaint and any other process in any other Action relating to the Acquisition, on behalf of itself or its property, by the delivery of copies of such process to such party in the same manner as notice is to be provided pursuant to ARTICLE IX. Nothing in this Section 9.9 shall affect the right of any party hereto to serve legal process in any other manner permitted by law.
9.9.3 EACH PARTY HEREBY WAIVES, TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAW, ANY RIGHT TO TRIAL BY JURY OF ANY ACTION (A) ARISING UNDER THIS AGREEMENT OR (B) IN ANY WAY CONNECTED WITH OR RELATED OR INCIDENTAL TO THE DEALINGS OF THE PARTIES IN RESPECT OF THIS AGREEMENT OR ANY OF THE TRANSACTIONS RELATED HERETO, IN EACH CASE, WHETHER NOW EXISTING OR HEREAFTER ARISING, AND WHETHER IN CONTRACT, TORT, EQUITY, OR OTHERWISE. EACH PARTY HEREBY FURTHER AGREES AND CONSENTS THAT ANY SUCH ACTION SHALL BE DECIDED BY COURT TRIAL WITHOUT A JURY AND THAT THE PARTIES MAY FILE A COPY OF THIS AGREEMENT WITH ANY COURT AS WRITTEN EVIDENCE OF THE CONSENT OF THE PARTIES TO THE WAIVER OF THEIR RIGHT TO TRIAL BY JURY.
9.10 Specific Performance. The Parties agree that irreparable damage would occur in the event that any of the provisions of this Agreement were not performed in accordance with their specific terms or were otherwise breached or threatened to be breached and that an award of money damages would be inadequate in such event. Accordingly, it is acknowledged that, prior to the valid termination of this Agreement pursuant to Section 7.1, the Parties shall be entitled to equitable relief, without proof of actual damages, including an injunction or order for specific performance to prevent breaches of this Agreement and to enforce specifically the terms and provisions of this Agreement, in addition to any other remedy to which they are entitled at law or in equity as a remedy for any such breach or threatened breach; provided, however, for the avoidance of doubt, under no circumstance shall Sen Lang be permitted or entitled to receive both a grant of specific performance that results in the consummation of the transactions contemplated by this Agreement and monetary damages, including any monetary damages in lieu of specific performance. Each Party further agrees that no other Party or any other Person shall be required to obtain, furnish or post any bond or similar instrument in connection with or as a condition to obtaining any remedy referred to in this Section 9.10, and each Party (a) irrevocably waives any right it may have to require the obtaining, furnishing or posting of any such bond or similar instrument and (b) agrees to cooperate fully in any attempt by the other Party or Parties in obtaining such equitable relief. Each Party further agrees that the only permitted objection that it may raise in response to any action for equitable relief is that it contests the existence of a breach or threatened breach of this Agreement.
A-58
9.11 Assignment. This Agreement shall be binding upon and inure to the benefit of the Parties named herein and their respective successors and permitted assigns. No Party may assign either this Agreement or any of its rights, interests, or obligations hereunder without the prior written approval of Buyer and Seller; provided, however, that Buyer may (i) assign any or all of its rights and interests hereunder to one or more of its Affiliates, (ii) designate one or more of its Affiliates to perform its obligations hereunder (in any or all of which cases Buyer nonetheless shall remain responsible for the performance of all of its obligations hereunder), and (iii) assign collaterally to any lender(s) its rights hereunder.
9.12 Expenses. Subject to the provisions of Section 7.2, all costs and expenses incurred in connection with this Agreement and the transactions contemplated hereby shall be paid by the party incurring such expenses, except that those expenses incurred in connection with filing, printing and mailing the Proxy Statement (including filing fees related thereto but excluding legal and accounting fees and expenses) and the fees and disbursements of any third-party service provider with respect thereto will be shared equally by Avalon and the Sen Lang Representative.
9.13 Severability. Any term or provision of this Agreement that is invalid or unenforceable in any situation in any jurisdiction shall not affect the validity or enforceability of the remaining terms and provisions hereof or the validity or enforceability of the offending term or provision in any other situation or in any other jurisdiction.
9.14 Release. In consideration of, among other things, the entry into this Agreement by Avalon, Sen Lang, and the Sen Lang Representative and the consummation of the transactions contemplated hereby and each Sen Lang Owner’s entitlement to receive, directly or indirectly, the Common Exchange Shares payable pursuant to this Agreement, effective as of the Closing, each Sen Lang Owner, on its own behalf and on behalf of such Sen Lang Owner’s successors, Affiliates, assigns, heirs, trustees, administrators and executors (each, a “Sen Lang Releasing Party”), hereby irrevocably releases and discharges each Acquired Company, and its direct and indirect equityholders and its past, present and future directors, officers, managers, employees, members, partners, shareholders, agents, attorneys, advisors, representatives, successors, and assigns (collectively, the “Released Parties”) from any and all debts, losses, costs, bonds, suits, actions, causes of action, liabilities, contributions, attorneys’ fees, interest, damages, punitive damages, expenses, claims, potential claims, counterclaims, cross-claims or demands, in law or in equity, asserted or unasserted, express or implied, known or unknown, matured or unmatured, contingent or vested, liquidated or unliquidated, of any kind or nature or description whatsoever, that the Sen Lang Releasing Party had, presently has or may hereafter have or claim or assert to have against any of the Released Parties arising on or prior to the Closing Date solely to the extent based upon such Sen Lang Owner’s capacity as a holder of equity, directly or indirectly, in any Acquired Company (collectively, the “Sen Lang Released Claims”). This release is intended to be a complete and general release with respect to the Sen Lang Released Claims, and specifically includes claims that are known, unknown, fixed, contingent or conditional, including breach of fiduciary duty, or claims arising under any federal, state, blue sky or local law dealing with any securities.
A-59
9.15 Governing Language; Translations. This Agreement has been negotiated and executed by the Parties in English. In the event of a conflict between different translations of these terms, the English translation will govern.
9.16 Voluntary Agreement. The Sen Lang Parties acknowledge and warrant that (a) they have been advised that (i) the law firm of Lowenstein Sandler LLP prepared this Agreement, (ii) in preparing this Agreement, the law firm of Lowenstein Sandler LLP represented only the interests of Avalon, and (iii) the interests of the Sen Lang Parties may be different from Avalon’s interests, (b) they have been afforded a reasonable opportunity to review this Agreement, to understand its terms and to discuss it with an attorney of their choice and (c) they knowingly and voluntarily enter into this Agreement.
ARTICLE X.
DEFINITIONS
10.1 Certain Definitions. For purpose of this Agreement, the following capitalized terms have the following meanings:
10.1.1 “Accounting Principles” means in accordance with generally accepted accounting principles as in effect at the date of the financial statement to which it refers or if there is no such financial statement, then as of the Closing Date, using and applying the same accounting principles, practices, procedures, policies and methods (with consistent classifications, judgments, elections, inclusions, exclusions and valuation and estimation methodologies) used and applied by the Acquired Companies in the preparation of the Sen Lang Financial Statements.
10.1.2 “Action” means any notice of noncompliance or violation, or any claim, demand, charge, action, suit, litigation, audit, settlement, complaint, stipulation, assessment or arbitration, or any request (including any request for information), inquiry, hearing, or proceeding, by or before any Governmental Authority.
10.1.3 “Acquired Companies” means Sen Lang and each of its Subsidiaries. Acquired Companies shall also include the OpCo and Senlang Lab.
10.1.4 “Affiliate” means, with respect to any Person, any other Person directly or indirectly Controlling, Controlled by, or under common Control with such Person.
10.1.5 “Ancillary Documents” means each agreement, instrument or document attached hereto as an Exhibit or executed in connection herewith.
10.1.6 “Applicable Law” means all laws, rules and regulations applicable to the Person, conduct, transaction, covenant, Ancillary Document or contract in question, including all applicable common law and equitable principles, all provisions of all applicable state, federal and foreign constitutions, statutes, rules, regulations, treaties, directives and orders of any Governmental Authority, and all orders, judgments and decrees of any Governmental Authority.
10.1.7 “Avalon Equity Financing” means an equity financing, through the issuance of equity interests of the OpCo, of no less than Two Hundred Million RMB (RMB200,000,000), in three (3) installments, the first (RMB67,000,000) to be paid in at the Closing, the second (RMB67,000,000) to be paid within three (3) months after the Closing, and the third (RMB66,000,000) to be paid within six (6) months after the Closing, on the terms and conditions approved by Avalon and set forth in the definitive financing documentation.
A-60
10.1.8 “Avalon Intellectual Property Rights” means and includes all Intellectual Property Rights relating to the development, manufacture, marketing, sale, licensing or maintenance of products, technologies or services by Avalon or its Subsidiaries or otherwise used in the conduct of the business of Avalon or its Subsidiaries.
10.1.9 “Avalon Per Share Price” means an amount equal the lower of: (i) the closing price of the Avalon Common Stock (as reflected on Nasdaq.com) immediately preceding the date of this Agreement; or (ii) the average closing price of the Avalon Common Stock (as reflected on Nasdaq.com) for the five Trading Days immediately preceding the date of this Agreement (calculated on a simple, not weighted average, basis). “Trading Day” means any day on which shares of Avalon Common Stock are actually traded on the principal securities exchange or securities market on which shares of Avalon Common Stock are then traded. All such determinations shall be appropriately adjusted for any stock dividend, stock split, stock combination, recapitalization or other similar transaction during such period.
10.1.10 “Business Day” means a day, other than a Saturday or Sunday, on which commercial banks in New York City are open for the general transaction of business.
10.1.11 “Cash” means, as of the Reference Time, the aggregate cash and cash equivalents of the Acquired Companies on hand or in bank accounts, including deposits in transit, minus the aggregate amount of outstanding and unpaid checks issued by or on behalf of the Acquired Companies as of such time.
10.1.12 “Code” means the Internal Revenue Code of 1986, as amended.
10.1.13 “Control” of a Person means the possession, directly or indirectly, of the power to direct or cause the direction of the management and policies of such Person, whether through the ownership of voting securities, by contract, or otherwise. “Controlled”, “Controlling” and “under common Control with” have correlative meanings. Without limiting the foregoing a Person (the “Controlled Person”) shall be deemed Controlled by (a) any other Person (i) owning beneficially, as meant in Rule 13d-3 under the Exchange Act, securities entitling such Person to cast ten percent (10%) or more of the votes for election of directors or equivalent governing authority of the Controlled Person or (ii) entitled to be allocated or receive ten percent (10%) or more of the profits, losses, or distributions of the Controlled Person; (b) an officer, director, general partner, partner (other than a limited partner), manager, or member (other than a member having no management authority that is not a Person described in clause (a) above) of the Controlled Person; or (c) a spouse, parent, lineal descendant, sibling, aunt, uncle, niece, nephew, mother-in-law, father-in-law, sister-in-law, or brother-in-law of an Affiliate of the Controlled Person or a trust for the benefit of an Affiliate of the Controlled Person or of which an Affiliate of the Controlled Person is a trustee.
A-61
10.1.14 “Control Claim” means any claim arising in whole or in part out of or resulting directly or indirectly from (whether or not involving a Third Party Claim): (a) the Control Documents becoming illegal, void, unenforceable or ineffective, as determined by Avalon in its sole and absolute discretion, under Applicable Law; (b) a competent authority challenging or prohibiting the collection and use of human genetic resources by the Acquired Companies; or (c) any unwinding of the Acquisition under Applicable Law or forced divestiture of the Sen Lang Shares under Applicable Law that frustrates the purpose of the Acquisition.
10.1.15 “Control Documents” means the documents attached hereto as Exhibit E.
10.1.16 “Encumbrance” means any mortgage, pledge, deed of trust, hypothecation, right of others, claim, security interest, encumbrance, burden, title defect, title retention agreement, lease, sublease, license, occupancy agreement, easement, covenant, condition, encroachment, voting trust agreement, interest, option, right of first offer, negotiation or refusal, proxy, lien, charge or other restriction or limitations of any nature whatsoever, including but not limited to such Encumbrances as may arise under any contract.
10.1.17 “Environmental Laws” means all Laws relating to the protection of the environment, to human health and safety, or to any Environmental activity, including (a) CERCLA, the Resource Conservation and Recovery Act, and the occupational safety and Health Act, or any equivalent law under the PRC (b) all other requirements pertaining to reporting, licensing, permitting, investigation or remediation of emissions, discharges, releases or threatened releases of Hazardous Materials into the air, surface water, groundwater or land, or relating to the manufacture, processing, distribution, use, sale, treatment, receipt, storage, disposal, transport or handling of Hazardous Materials and (c) all other requirements pertaining to the protection of the health and safety of employees or the public.
10.1.18 “Executory Period” means the period beginning at 12:01a.m. on the Effective Date and ending at the Closing.
10.1.19 “Governmental Authority” means any federal, state, local, foreign or other governmental, quasi-governmental or administrative body, instrumentality, department or agency or any court, tribunal, administrative hearing body, arbitration panel, commission, or other similar dispute-resolving panel or body.
10.1.20 “Hazardous Materials” means any substance that: (a) is or contains asbestos, urea formaldehyde foam insulation, polychlorinated biphenyls, petroleum or petroleum-derived substances or wastes, radon as or related materials (b) requires investigation, removal or remediation under any Environmental Law, or is defined, listed or identified as a “hazardous waste” or “hazardous substance” thereunder, or (c) is toxic, explosive, corrosive, flammable, infectious, radioactive, carcinogenic, mutagenic, or otherwise hazardous and is regulated by any Governmental Authority or Environmental Law.
10.1.21 “Income Tax” means any Tax computed in whole or in part based on or by reference to net income and any alternative, minimum, accumulated earnings or personal holding company Tax (including all interest and penalties thereon and additions thereto).
A-62
10.1.22 “Income Tax Return” means any return, report, declaration, form, claim for refund or information return or statement relating to Income Taxes, including any schedule or attachment thereto, and including any amendment thereof.
10.1.23 “Indebtedness” of any Person means, without duplication, (a) all indebtedness of such Person for borrowed money (including the outstanding principal and accrued but unpaid interest), (b) all obligations for the deferred purchase price of property or services (other than trade payables incurred in the ordinary course of business), (c) any other indebtedness of such Person that is evidenced by a note, bond, debenture, credit agreement or similar instrument, (d) all obligations of such Person under leases that should be classified as capital leases in accordance with generally accepted accounting principles, (e) all obligations of such Person for the reimbursement of any obligor on any line or letter of credit, banker’s acceptance, guarantee or similar credit transaction, in each case, that has been drawn or claimed against, (f) all obligations of such Person in respect of acceptances issued or created, (g) all interest rate and currency swaps, caps, collars and similar agreements or hedging devices under which payments are obligated to be made by such Person, whether periodically or upon the happening of a contingency, (h) all obligations secured by an Encumbrance on any property of such Person, (i) any premiums, prepayment fees or other penalties, fees, costs or expenses associated with payment of any Indebtedness of such Person, (j) all obligation described in clauses (a) through (i) above of any other Person which is directly or indirectly guaranteed by such Person or which such Person has agreed (contingently or otherwise) to purchase or otherwise acquire or in respect of which it has otherwise assured a creditor against loss, and (k) any unpaid liabilities of the Acquired Companies for Taxes, including such Taxes not yet accrued or due and payable but attributable to Tax periods (or portions thereof) ending on or prior to the Closing.
10.1.24 “Intellectual Property Rights” means all of the following in any jurisdiction throughout the world: (i) patents, patent applications (including divisionals, continuations, continuations-in-part, reissues, reexaminations and extensions thereof) and patent disclosures (including design patents, design rights, utility models and other similar registered rights); (ii) trademarks, service marks, trade dress, trade names, corporate names, logos and slogans (and all translations, adaptations, derivations and combinations of the foregoing) and Internet domain names, together with similar designations or source or origin and all goodwill associated with each of the foregoing; (iii) copyrights and copyrightable works, works of authorship, databases and designs; (iv) registrations and applications and any other similar filings submitted to, issued by or recorded with any Governmental Authority for any of the foregoing; (v) all trade secrets and other confidential or proprietary information, know-how and inventions (whether or not patentable or reduced to practice), including algorithms, processes, techniques, methods, formulations, customer, supplier or subscriber lists, plans, business and marketing materials and compounds, discoveries, technologies, protocols, formulae, compositions, industrial models, architectures, layouts, designs, drawings, specifications, methodologies, ideas, research and development, pricing and cost information; (vi) software and firmware of any type, including rights in computer software, application programming interfaces, development kits, libraries and tools, data and databases (including source code, executable code, binary code, and documentation); (vii) data, data-sets and databases; and (viii) all other intellectual property, industrial property and proprietary rights and assets of any kind or nature means and includes rights relating to patents, trademarks, service marks, trade names, copyrights, mask works, inventions, processes, trade secrets, know-how, confidentiality agreements, consulting agreements, software and any documentation.
A-63
10.1.25 “Person” means an individual, corporation, partnership (including a general partnership, limited partnership or limited liability partnership), limited liability company, association, trust or other entity or organization, including a government, domestic or foreign, or political subdivision thereof, or an agency or instrumentality thereof.
10.1.26 “PRC” means the People’s Republic of China.
10.1.27 “Pro Rata Share” means, with respect to each Sen Lang Shareholder, a fraction, expressed as a percentage, equal to (i) the portion of the Common Exchange Shares payable by Avalon to such Sen Lang Shareholder in accordance with the terms of this Agreement, divided by (ii) the total Common Exchange Shares payable by Avalon to all Sen Lang Shareholders in accordance with the terms of this Agreement. All issuances of Avalon Common Stock shall be rounded to the nearest whole share.
10.1.28 “Reference Time” means 12:01 A.M. New York time on the Closing Date.
10.1.29 “Release” means any releasing, disposing, discharging, injecting, spilling, leaking, leaching, pumping, dumping, emitting, escaping, emptying, seeing, dispersal, leeching, migration, transporting, placing and the like, including the moving of any materials through, into or upon, any land, soil, surface water, ground water or air, or otherwise entering into the environment.
10.1.30 “Representatives” means, as to any Person, such Person’s Affiliates and the respective managers, directors, officers, employees, independent contractors, consultants, advisors (including financial advisors, counsel and accountants), agents and other legal representatives of such Person or its Affiliates.
10.1.31 “Return” means any return, report, declaration, form, election, claim for refund or information return or statement relating to Taxes, including any schedule or attachment thereto, and including any amendment thereof.
10.1.32 “Sanctioned Person” means any individual or entity that is, or is owned fifty percent (50%) or more, directly or indirectly, individually or in the aggregate, or is controlled, by any party that (i) is the subject of any Sanctions Laws, including by being designated on, any list of restricted parties maintained by the U.S. Government, the United Nations Security Council, the European Union, Her Majesty’s Treasury or any other relevant sanctions authority, including, without limitation, the Office of Foreign Assets Control’s Specially Designated Nationals and Blocked Persons List, list of Foreign Sanctions Evaders, and Sectoral Sanctions Identifications List, the U.S. Department of Commerce’s Denied Persons List and Entity List, and the U.S. Department of State’s Debarred List, (ii) is located, organized or resident in a country or territory that is, or whose government is, the subject of comprehensive territorial Sanctions Laws, including, without limitation, Crimea region of Ukraine, Cuba, Iran, North Korea, and Syria, or (iii) is other the subject of any other prohibitions which make it unlawful for a U.S. person to transact with the entity under applicable laws.
10.1.33 “SEC” means the U.S. Securities and Exchange Commission (or any successor Governmental Authority).
10.1.34 “Securities Act” means the Securities Act of 1933, as amended.
10.1.35 “Sen Lang Intellectual Property Rights” means and includes all Intellectual Property Rights used in the development, manufacture, marketing, sale, licensing or maintenance of products, technologies or services by the Acquired Companies or otherwise used in the conduct of the business of the Acquired Companies.
A-64
10.1.36 “Sen Lang Material Adverse Effect” means, with respect to any event, occurrence, matter, failure of event or occurrence, change, effect, state of affairs, breach, default, violation, fine, penalty or failure to comply (each, a “Circumstance”), individually or taken together with all other Circumstances contemplated by or in connection with any or all of the representations and warranties made in this Agreement, a material adverse effect on the business, assets (including intangible assets), liabilities (contingent or otherwise), financial condition, results of operations or prospects of the Acquired Companies, taken as a whole; provided, however, that the term “Sen Lang Material Adverse Effect” shall not be deemed to include the impact of: (A) the implementation of changes in U.S. generally accepted accounting principles; (B) actions and omissions of the Acquired Companies taken or permitted with the prior written consent of Avalon after the date hereof; (C) expenses reasonably incurred by the Acquired Companies in consummating the transactions contemplated by this Agreement; (D) changes in the general economic or financial market conditions; (E) any occurrence, condition, change, event or effect that affects the biotechnology industry generally; and (F) acts of God, war, acts of terrorism, epidemics, pandemics, quarantines, injunctions or restraining orders, failure of any public authority or governmental body or agency to issue any permit or license, or generalized lack of raw materials or energy.
10.1.37 “SOX” means the U.S. Sarbanes-Oxley Act of 2002, as amended.
10.1.38 “Subsidiary” means, with respect to any Person, any corporation, partnership, association or other business entity of which (i) if a corporation, a majority of the total voting power of shares of stock entitled (without regard to the occurrence of any contingency) to vote in the election of directors, managers or trustees thereof is at the time owned or controlled, directly or indirectly, by that Person or one or more of the other Subsidiaries of that Person or a combination thereof, or (ii) if a partnership, association or other business entity, a majority of the partnership or other similar ownership interests thereof is at the time owned or controlled, directly or indirectly, by any Person or one or more Subsidiaries of that Person or a combination thereof. For purposes hereof, a Person or Persons will be deemed to have a majority ownership interest in a partnership, association or other business entity if such Person or Persons will be allocated a majority of partnership, association or other business entity gains or losses or will be or control the managing director, managing member, general partner or other managing Person of such partnership, association or other business entity. A Subsidiary of a Person will also include any variable interest entity which is consolidated with such Person under applicable accounting rules
10.1.39 “Tax” means (i) any and all U.S. and non-U.S. federal, state, provincial, local and other taxes, charges, duties, contributions, fees, levies or other similar assessments or liabilities (including income, receipts, ad valorem, value added, excise, real or personal property, sales, occupation, service, stamp, transfer, registration, natural resources, severance, premium, windfall or excess profits, environmental, customs duties, use, licensing, escheat, unclaimed or abandoned property, withholding, employment, social security, unemployment, disability, payroll, share, capital, surplus, alternative, minimum, add-on minimum, estimated, franchise or any other taxes, charges, fees, levies or other similar assessments or liabilities of any kind whatsoever and denominated by any name whatsoever), whether computed on a separate, consolidated, unitary or combined basis or in any other manner, and includes any interest, fines, penalties, assessments, deficiencies or additions thereto, whether disputed or not, (ii) any and all liability for amounts described in clause (i) of any member of an affiliated, consolidated, combined or unitary group of which the Acquired Companies (or any predecessor of any of the foregoing) is or was a member on or prior to the Closing Date, including pursuant to Treasury Regulations Section 1.1502-6 or any analogous or similar state, local or non-U.S. Law or regulation, and (iii) any sales, use, real property transfer, stamp, stock transfer or other similar transfer Taxes imposed on Avalon or any Acquired Company in connection with the Acquisition or the other transactions contemplated by this Agreement and any China Enterprise Income Tax and any other similar Taxes imposed on any party in connection with the Acquisition or the other transactions contemplated by this Agreement.
[Remainder of Page Intentionally Left Blank]
A-65
IN WITNESS WHEREOF, each of the Parties has caused this Agreement to be duly executed on its behalf as of the day and year first above written.
| AVALON GLOBOCARE CORP. | ||
| By: | /s/ Dr, David Jin | |
| Name: Dr, David Jin | ||
| Title: CEO | ||
| LONLON BIOTECH LTD. | ||
| By: | /s/ Ding Wei | |
| Name: Ding Wei | ||
| Title: Director | ||
| [SEN LANG PARTY SIGNATURE PAGES TO BE PROVIDED SEPARATELY] | ||
[Signature Page Sen Lang Share Purchase Agreement]
Exhibit A
Sen Lang Owners
Exh. A - 1
Exhibit B
Form of AI Letter
[See attached]
Exh. B - 1
Exhibit C
Form of Letter of Transmittal
[See attached]
Exh. C - 1
Exhibit D
Form of Escrow Agreement
[See attached]
Exh. D - 1
Exhibit E
List of Control Documents
[See attached]
Exh. E - 1