
Exhibit 99.1

www.avalon - globocare.com Nasdaq: AVCO Corporate Presentation May 2022

Certain statements contained in this presentation may constitute “forward - looking statements”, which provide current expectations of future events based on certain assumptions and include any statement that does not directly relate to any historical or current fact . Actual results may differ materially from those indicated by such forward - looking statements as a result of various important factors as disclosed in our filings with the Securities and Exchange Commission located at their website ( http : //www . sec . gov ) . In addition to these factors, actual future performance, outcomes, and results may differ materially because of more general factors including (without limitation) general industry and market conditions and growth rates, economic conditions, and governmental and public policy changes . The forward - looking statements included in this presentation represent the Company's views as of the date of this presentation and these views could change . However, while the Company may elect to update these forward - looking statements at some point in the future, the Company specifically disclaims any obligation to do so . These forward - looking statements should not be relied upon as representing the Company's views as of any date subsequent to the date of this presentation . Forward - Looking Statements

Mission: Avalon GloboCare Corp. (Nasdaq: AVCO) is a clinical - stage biotechnology company dedicated to develop and deliver innovative and transformative CellTech with multifunctional convergence in cellular technologies/therapeutics, precision diagnostics and intellectual property in the field of immuno - oncology and cellular medicines History: Founded in 2016; successfully uplisted to Nasdaq in December 2018; Headquarters in Freehold, NJ; approx. 20 FTEs (including subsidiaries) Core Technology Platforms: - Cellular Immunotherapies including CAR - T, mRNA - based FLASH CAR TM - Avalon Clinical - Grade Tissue - Specific Exosomes ( ACTEX TM ) - AVA - Trap TM QTY Code - derived Decoy Receptors / Hemofiltartion System / Novel Therapeutic Targets - Precision Companion Diagnostics Corporate Overview and Highlights Nasdaq: AVCO 3

Corporate Overview and Highlights Leveraging platform through strategic partnering opportunities with academia and industries complementary to our R&D programs - Massachusetts Institute of technology - University of Pittsburgh Medical Center - Arbele Limited - HydroPeptide, LLC Strong Intellectual Property Portfolio: Underpinning Avalon’s R&D pipeline is a broad and deep intellectual property portfolio, covering a number of key enabling technologies and addressing multi - billion dollars of unmet CellTech market worldwide Nasdaq: AVCO 4

Nasdaq: AVCO 5 Senior Management, Board of Directors, Advisors Robert S. Langer, Sc.D. -- Massachusetts Institute of Technology; David H. Koch Institute Professor Yen - Michael Hsu, M.D., Ph.D.. -- University of Pittsburgh Medical Center, Chief of Cellular Therapy Peihua Peggy Lu, M.D -- Executive President, Lu Daopei Hospital Dongfang Liu, Ph.D. -- Rutgers New Jersey Medical School; Director of Immunoassay Program Hongxing Liu, M.D., M.S. -- Lu Daopei Hematology Institute, Executive President Uwe B. Sletyr, Ph.D. -- Professor Emeritus, University of Natural Resources and Life Sciences Full Member, Austrian Academy of Sciences Meng Li COO Former WPP Group’s company executive Luisa Ingargiola, MHA CFO, Former CFO and BoD of several U.S. Public companies Anna Azvolinsky, Ph.D. Head, Media and Communication Team of Our Subsidiaries David Jin, M.D., Ph.D. CEO, Avactis (JV with Arbele) John Luk, M.D., D.Sc. President, Avactis Steven Sukel, J.D. Managing Director, Avalon RT9 Properties, LLC Lucy Lu President, Nanjing Epicon Biotech co., Ltd. Professor Daopei Lu, M.D. Scientific Founder Scientific & Clinical Advisory Board Senior Management Team Board of Directors Daniel Lu Chairman of the Board Congressman Billy Tauzin Director; Former U.S. Congressman; Former President of PhRMA David Jin, M.D., Ph.D. Director, CEO, President Tevi Troy, Ph.D. Director; Chairman of Nomination/Governance Committees Former Deputy Director of U.S. Human Health Services Yancen Lu Director, Chairman of Compensation Committee Founder and Managing Director, Pagoda Tree Group Steven Sanders, J.D. Director, Co - Chari of Compensation Committee Founder of Ortoli Rosenstadt Law Firm, NYC William Stilley Director, Chairman of Audit Committee CEO, Adial Pharmaceuticals (Nasdaq:ADIL) Yue Charles Li Director, M&A Taskforce David Jin, M.D., Ph.D. CEO, President, Co - founder, BoD U.S. Licensed Physician; Former Medical Resident, Fellow and Faculty Member at Weill Cornell Medicine and New York - Presbyterian Hospital; Senior Clinician - Scientist at Ansary Stem Cell Institute; Former CMO of BioTime Inc. and OncoCyte Corporation Meng Li COO Former WPP Group’s company executive Luisa Ingargiola, MHA CFO, Former CFO and BoD of several U.S. Public companies Anna Azvolinsky, Ph.D. Head, Media and Communication Anna Azvolinsky, Ph.D. Head, Media and Communication Angela An Vice President, APAC Business Development

Key Differentiators
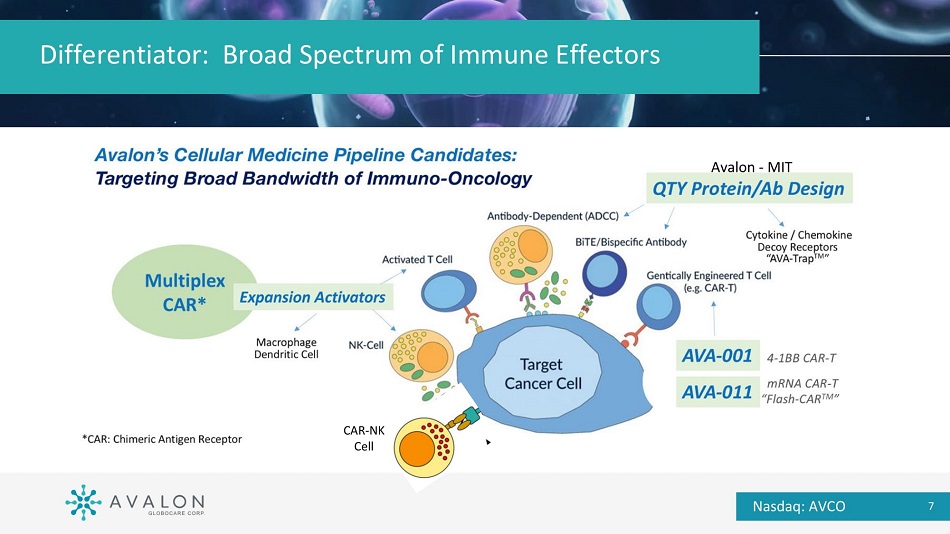
Differentiator: Broad Spectrum of Immune Effectors CAR - NK Cell Nasdaq: AVCO 7 Avalon - MIT
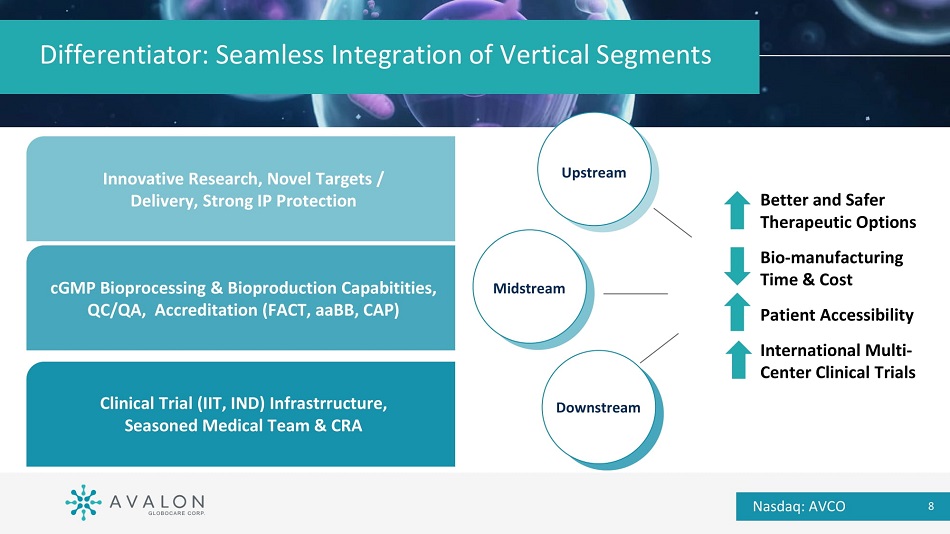
Differentiator: Seamless Integration of Vertical Segments Innovative Research, Novel Targets / Delivery, Strong IP Protection cGMP Bioprocessing & Bioproduction Capabitities, QC/QA, Accreditation (FACT, aaBB, CAP) Clinical Trial (IIT, IND) Infrastrructure, Seasoned Medical Team & CRA Upstream Midstream Downstream Better and Safer Therapeutic Options Bio - manufacturing Time & Cost Patient Accessibility International Multi - Center Clinical Trials Nasdaq: AVCO 8

CAR - T and FLASH - CAR TM Cell Therapy
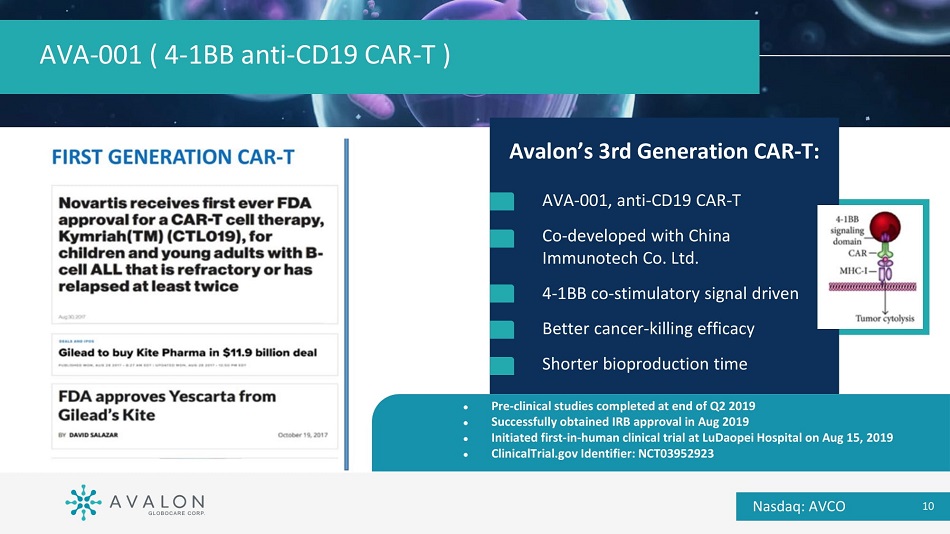
AVA - 001 ( 4 - 1BB anti - CD19 CAR - T ) Avalon’s 3rd Generation CAR - T: AVA - 001, anti - CD19 CAR - T Co - developed with China Immunotech Co. Ltd. 4 - 1BB co - stimulatory signal driven Better cancer - killing efficacy Shorter bioproduction time ● Pre - clinical studies completed at end of Q2 2019 ● Successfully obtained IRB approval in Aug 2019 ● Initiated first - in - human clinical trial at LuDaopei Hospital on Aug 15, 2019 ● ClinicalTrial.gov Identifier: NCT03952923 Nasdaq: AVCO 10
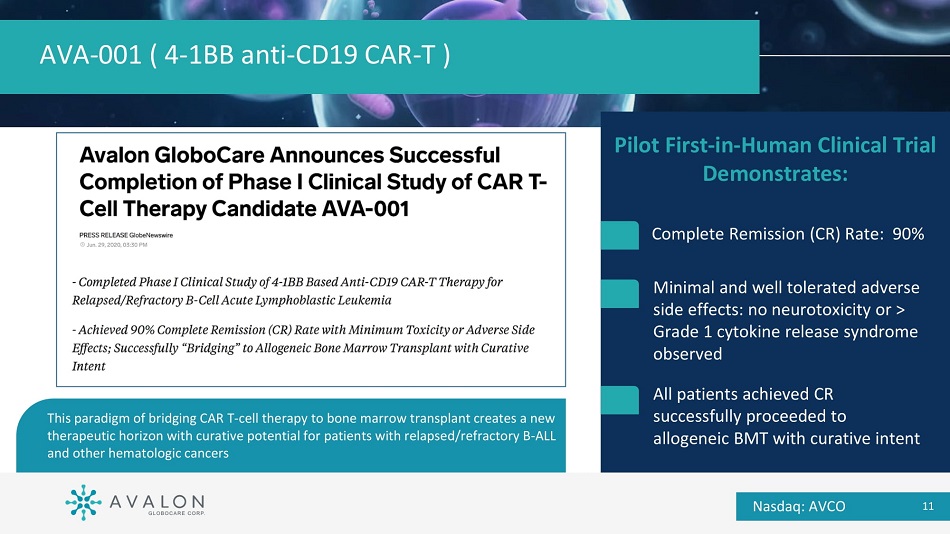
Pilot First - in - Human Clinical Trial Demonstrates: Complete Remission (CR) Rate: 90% Minimal and well tolerated adverse side effects: no neurotoxicity or > Grade 1 cytokine release syndrome observed All patients achieved CR successfully proceeded to allogeneic BMT with curative intent AVA - 001 ( 4 - 1BB anti - CD19 CAR - T ) This paradigm of bridging CAR T - cell therapy to bone marrow transplant creates a new therapeutic horizon with curative potential for patients with relapsed/refractory B - ALL and other hematologic cancers Nasdaq: AVCO 11

FLASH - CAR TM - - A Multiplex, mRNA - CAR Platform Nasdaq: AVCO 12
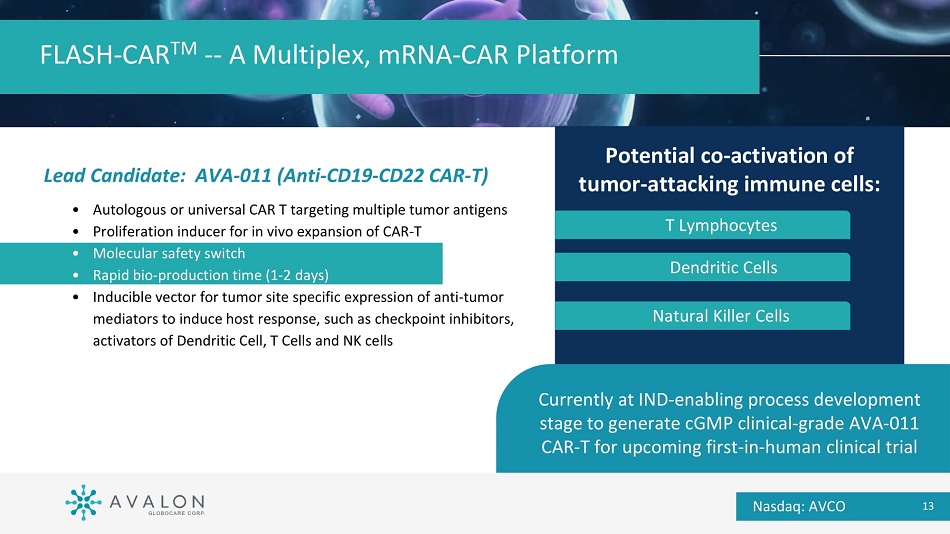
Lead Candidate: AVA - 011 (Anti - CD19 - CD22 CAR - T) • Autologous or universal CAR T targeting multiple tumor antigens • Proliferation inducer for in vivo expansion of CAR - T • Molecular safety switch • Rapid bio - production time (1 - 2 days) • Inducible vector for tumor site specific expression of anti - tumor mediators to induce host response, such as checkpoint inhibitors, activators of Dendritic Cell, T Cells and NK cells FLASH - CAR TM - - A Multiplex, mRNA - CAR Platform Currently at IND - enabling process development stage to generate cGMP clinical - grade AVA - 011 CAR - T for upcoming first - in - human clinical trial Potential co - activation of tumor - attacking immune cells: T Lymphocytes Dendritic Cells Natural Killer Cells Nasdaq: AVCO 13
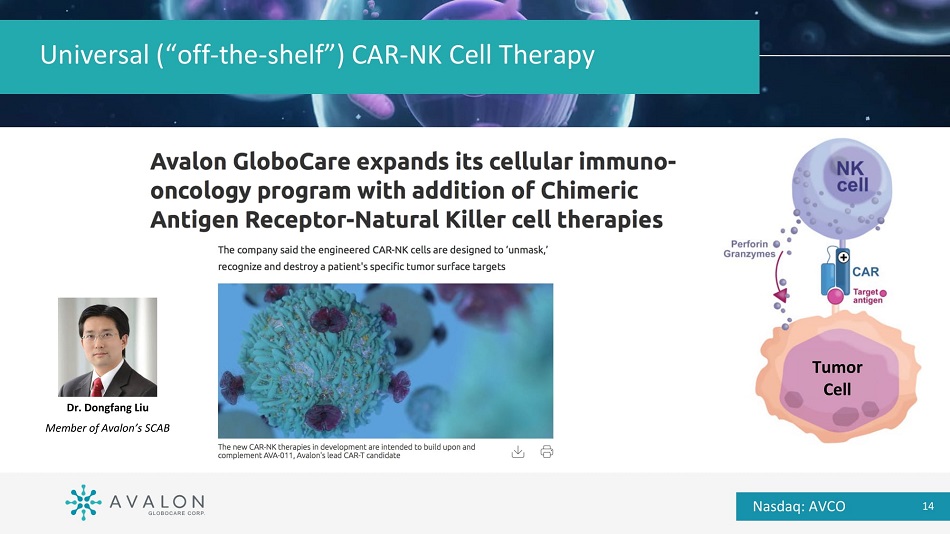
Universal (“off - the - shelf”) CAR - NK Cell Therapy Tumor Cell Dr. Dongfang Liu Member of Avalon’s SCAB Nasdaq: AVCO 14
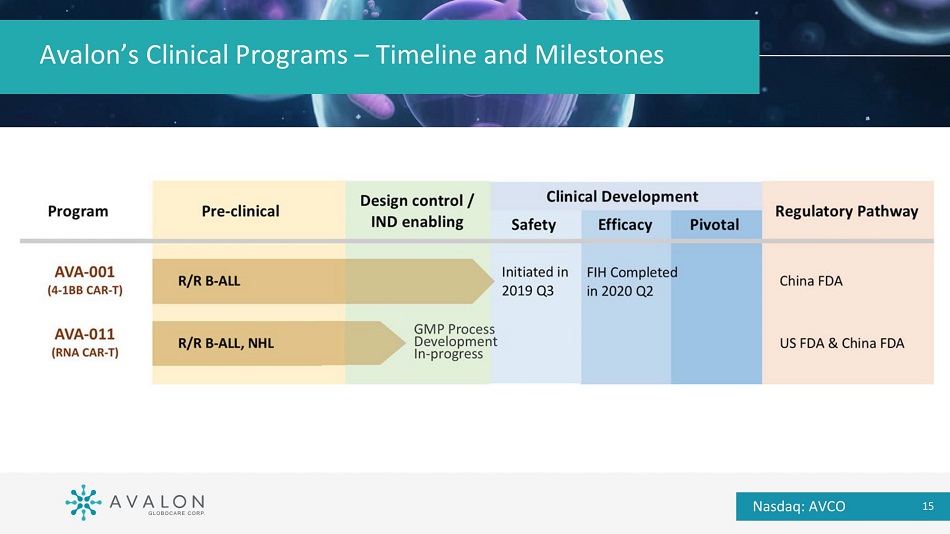
Avalon’s Clinical Programs – Timeline and Milestones Nasdaq: AVCO 15

Strategic Partnerships

Avalon Academic - Industry Partnership QTY Protein Design Code to Develop Decoy Cytokine & Chemokine Receptors to Combat “Cytokine Storm” and Cancer Metastasis (AVA - Trap Ρ ) Pls: Shuguang Zhang, Ph.D. Robert Langer, Sc.D. Process Development of Clinical - grade AVA - 011 (Flash - CAR Ρ ) and ACTEX Ρ (Exosome - based Technology Co - development of Point - of - Care Automated PD for Cellular Therapy (PMAPsys Ρ ) Pl: Yen - Michael Hsu, M.D., Ph.D. Nasdaq: AVCO 17
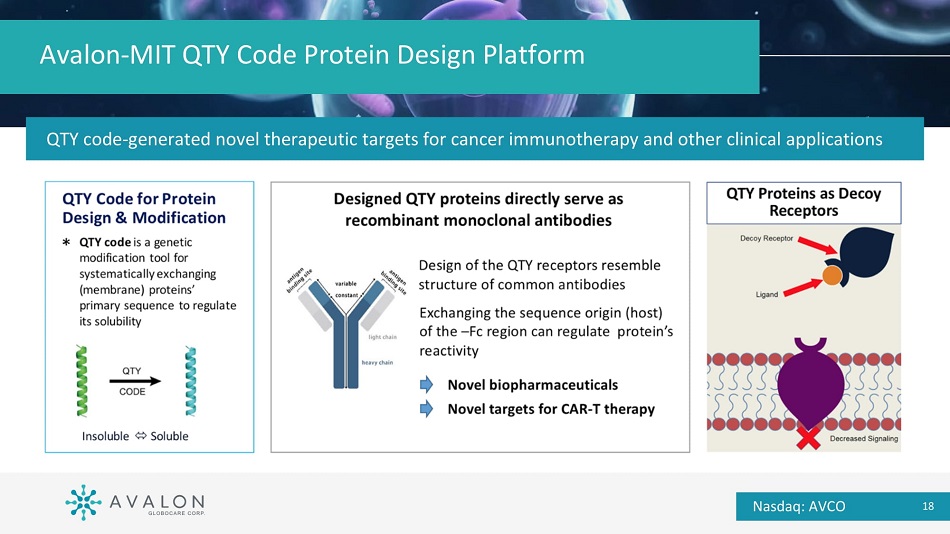
Avalon - MIT QTY Code Protein Design Platform QTY code - generated novel therapeutic targets for cancer immunotherapy and other clinical applications Nasdaq: AVCO 18
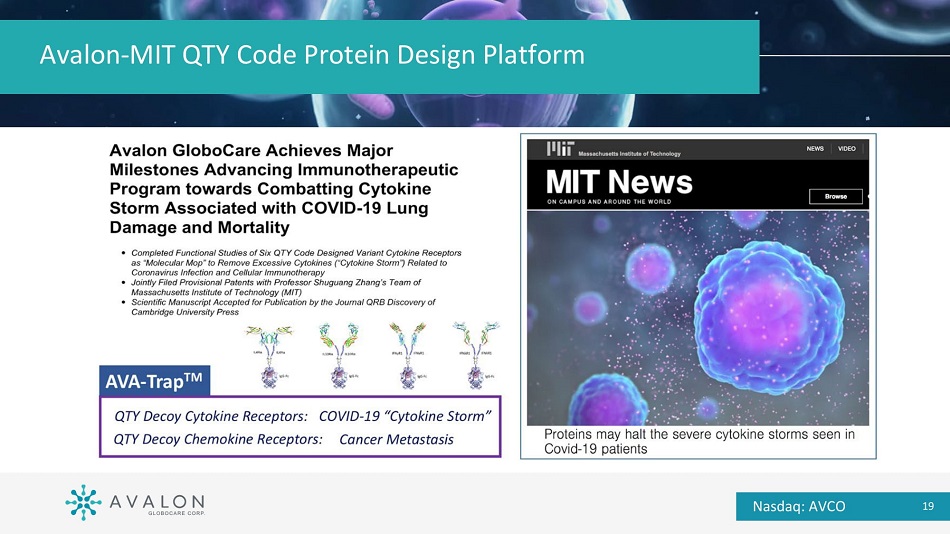
Avalon - MIT QTY Code Protein Design Platform Nasdaq: AVCO 19

Avalon ACTEX TM Platform (Avalon Clinical - grade Tissue - specific Exosomes) Clinical - Grade Stem/Progenitor Cells Bio - production ACTEX Ρ (Avalon Clinical - Grade Tissue - Specific Exosomes) Accreditation/Standardization Infrastructures Nasdaq: AVCO 20

Strategic Partnership with HydroPeptide Q1 of 2023 Nasdaq: AVCO 21
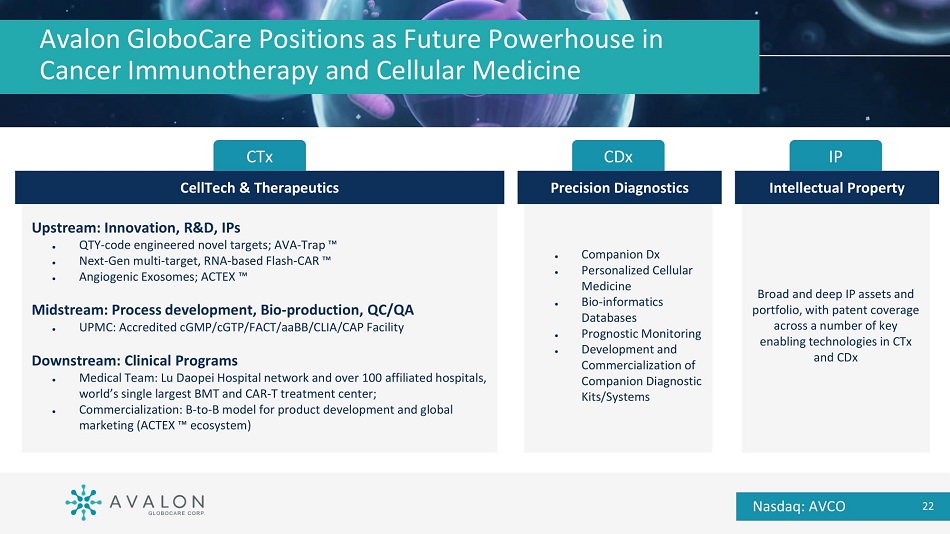
Broad and deep IP assets and portfolio, with patent coverage across a number of key enabling technologies in CTx and CDx Avalon GloboCare Positions as Future Powerhouse in Cancer Immunotherapy and Cellular Medicine IP Intellectual Property ● Companion Dx ● Personalized Cellular Medicine ● Bio - informatics Databases ● Prognostic Monitoring ● Development and Commercialization of Companion Diagnostic Kits/Systems CDx Precision Diagnostics Upstream: Innovation, R&D, IPs ● QTY - code engineered novel targets; AVA - Trap Ρ ● Next - Gen multi - target, RNA - based Flash - CAR Ρ ● Angiogenic Exosomes; ACTEX Ρ Midstream: Process development, Bio - production, QC/QA ● UPMC: Accredited cGMP/cGTP/FACT/aaBB/CLIA/CAP Facility Downstream: Clinical Programs ● Medical Team: Lu Daopei Hospital network and over 100 affiliated hospitals, world’s single largest BMT and CAR - T treatment center; ● Commercialization: B - to - B model for product development and global marketing (ACTEX Ρ ecosystem) CTx Nasdaq: AVCO 22 CellTech & Therapeutics

Corporate Council, American Society of Transplantation and Cellular Therapy (ASTCT) Nasdaq: AVCO 23

www.avalon - globocare.com Nasdaq: AVCO Contact: Corporate Address: Avalon Executive Center 4400 Route 9 South, Suite 3100 Freehold, New Jersey 07728, USA Phone: +1 – 732 - 780 - 4400 Fax: +1 – 732 - 780 - 5600 Website: www.avalon - globocare.com IR Email: avco@crescendo - ir.com Thank You