UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
For the fiscal year ended
OR
Commission file number:

(Name of registrant as specified in its charter)
| (State or other jurisdiction of | (I.R.S. Employer | |
| incorporation or organization) | Identification No.) |
| (Address of principal executive offices) | (Registrant’s telephone number) |
SECURITIES REGISTERED PURSUANT TO SECTION 12(b) OF THE EXCHANGE ACT:
| Title of each Class: | Trading Symbol | Name of Each Exchange | ||
| The |
SECURITIES REGISTERED PURSUANT TO SECTION 12(g) OF THE EXCHANGE ACT:
None.
Indicate by check mark if the Registrant is a
well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐
Indicate by check mark if the Registrant is not
required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐
Indicate by check mark whether the Registrant
(1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12
months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements
for the past 90 days.
Indicate by check mark whether the registrant
has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding
12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| ☒ | Smaller reporting company | ||
| Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant
has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial
reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or
issued its audit report. Yes ☐ No
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant
is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No
As of June 30, 2022, the last business day of
the Registrant’s most recently completed second fiscal quarter, the market value of our common stock held by non-affiliates was
approximately $
The number of shares of the Registrant’s
common stock, $0.0001 par value per share, outstanding as of March 30, 2023, was
Documents incorporated by reference: NONE
TABLE OF CONTENTS
i
Forward-Looking Statements
CERTAIN STATEMENTS IN THIS ANNUAL REPORT ON FORM 10-K MAY CONSTITUTE “FORWARD LOOKING STATEMENTS”. WHEN THE WORDS “BELIEVES,” “EXPECTS,” “PLANS,” “PROJECTS,” “ESTIMATES,” “OBJECTIVES,” “MAY,” “MIGHT,” “PREDICT,” “TARGET,” “POTENTIAL,” “WILL,” “WOULD,” “COULD,” “SHOULD,” “CONTINUE,” AND SIMILAR EXPRESSIONS ARE USED, THEY IDENTIFY FORWARD-LOOKING STATEMENTS. THESE FORWARD-LOOKING STATEMENTS ARE BASED ON MANAGEMENT’S CURRENT BELIEFS AND ASSUMPTIONS AND INFORMATION CURRENTLY AVAILABLE TO MANAGEMENT AND INVOLVE KNOWN AND UNKNOWN RISKS, UNCERTAINTIES AND OTHER FACTORS WHICH MAY CAUSE THE ACTUAL RESULTS, PERFORMANCE OR ACHIEVEMENTS OF THE COMPANY TO BE MATERIALLY DIFFERENT FROM ANY FUTURE RESULTS, PERFORMANCE OR ACHIEVEMENTS EXPRESSED OR IMPLIED BY THESE FORWARD-LOOKING STATEMENTS. INFORMATION CONCERNING FACTORS THAT COULD CAUSE OUR ACTUAL RESULTS TO DIFFER MATERIALLY FROM THESE FORWARD-LOOKING STATEMENTS CAN BE FOUND IN OUR PERIODIC REPORTS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION. YOU SHOULD READ THIS ANNUAL REPORT ON FORM 10-K AND THE DOCUMENTS THAT WE HAVE FILED AS EXHIBITS TO THIS ANNUAL REPORT ON FORM 10-K COMPLETELY. WE UNDERTAKE NO OBLIGATION TO PUBLICLY RELEASE REVISIONS TO THESE FORWARD-LOOKING STATEMENTS TO REFLECT FUTURE EVENTS OR CIRCUMSTANCES OR REFLECT THE OCCURRENCE OF UNANTICIPATED EVENTS, EXCEPT AS REQUIRED BY APPLICABLE LAW.
Unless otherwise indicated, references to “we,” “us,” “our,” “Company,” or “Avalon” mean Avalon GloboCare Corp. and its subsidiaries, and references to “fiscal” mean the Company’s fiscal year ended December 31. References to the “parent company” mean Avalon GloboCare Corp.
ii
PART I
ITEM 1. BUSINESS
Overview
We are a clinical-stage, vertically integrated, leading CellTech bio-developer dedicated to advancing and empowering innovative and transformative immune effector cell therapy and laboratory services. Through our membership interest in Lab Services MSO (“Lab Services”), we plan to focus on precision diagnostics along with toxicology and wellness testing. Through our subsidiary structure with unique integration of verticals from innovative R&D to automated bioproduction and accelerated clinical development, we are establishing a leading role in the fields of cellular immunotherapy (including CAR-T), and laboratory services.
Laboratory Services is focused on delivering high quality services related to toxicology and wellness testing and provides a broad portfolio of diagnostic tests including drug testing, toxicology, and a broad array of test services, from general bloodwork to anatomic pathology, and urine toxicology. Specific capabilities include STAT blood testing, qualitative drug screening, genetic testing, urinary testing, sexually transmitted disease testing and more. The panels that we test for are thyroid panel, comprehensive metabolic panel, kidney profile, liver function tests, and other individual tests. Through Laboratory Services, we use fast, accurate, and efficient equipment to provide practitioners with the tools to quickly determine if a patient is following their designated treatment plan. In most instances, we are able to provide a practitioner with qualitative drug class results the same day the sample is received. We provide an extensive chemistry test menu that gives physicians the information to better treat their patients and maintain their overall wellness and have developed a premier reputation for customer service and fast turnaround times in the industry.
We are also focused on achieving and fostering seamless integration of unique verticals to bridge and accelerate innovative research, bio-process development, clinical programs and product commercialization. Avalon’s upstream innovative research includes:
| ● | Novel therapeutic and diagnostic targets development utilizing QTY-code protein design technology with Massachusetts Institute of Technology (MIT) including using the QTY code protein design technology for development of novel therapeutic and diagnostic targets. |
| ● | Co-development of next generation, mRNA-based (Flash-CARTM) CAR-T, CAR-NK and other immune effector cell therapeutic modalities with Arbele Limited. |
Avalon’s midstream bio-processing and bio-production facility is affiliated with the University of Pittsburgh Medical Center where our leading candidate AVA-011, as described below, is undergoing process development to generate clinical grade CAR-T cells for upcoming clinical trial in the US.
Avalon’s downstream medical team and facility consists of top-rated affiliated hospital network and experts specialized in hematology, oncology, cellular immunotherapy, hematopoietic stem/progenitor cell transplant, as well as regenerative therapeutics. Our major clinical programs include:
| ● | AVA-001: Avalon has initiated its first-in-human clinical trial of CD19 CAR-T candidate, AVA-001 in August 2019 at the Hebei Yanda Lu Daopei Hospital and Beijing Lu Daopei Hospital in China (the world’s single largest CAR-T treatment network for the indication of relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL). The AVA-001 candidate (co-developed with China Immunotech Co. Ltd) is characterized by the utilization of 4-1BB (CD137) co-stimulatory signaling pathway, conferring a strong anti-cancer activity during pre-clinical study. It also features a shorter bio-manufacturing time which leads to the advantage of prompt treatment to patients where timing is important related hematologic malignancies. We have successfully completed the first-in-human clinical trial of our AVA-001 anti-CD19 CAR-T cell therapy as a bridge to allogeneic bone marrow transplantation for patients with relapsed/refractory B-ALL at the Lu Daopei Hospital (registered clinical trial number NCT03952923) with excellent efficacy (90% complete remission rate) and minimal adverse side effects. We are currently expanding the indication and plan to recuit patients in the USA for AVA-001 to include both relapsed/refractory B-ALL and non-Hodgkin lymphoma patients. |
1
| ● | AVA-011 and FLASH-CAR™: Avalon advanced its next generation immune cell therapy using mRNA-based, non-viral FLASH-CAR™ technology co-developed with our strategic partner Arbele Limited. The adaptable FLASH-CAR™ platform can be used to create personalized cell therapy from a patient’s own cells, as well as off-the-shelf cell therapy from a universal donor. Our leading candidate, AVA-011, is currently at process development stage to generate clinical-grade cell-therapy products for subsequent clinical studies. In July 2021, we and the University of Pittsburgh of the Commonwealth System of Higher Education (the “University”) entered into a Corporate Research Agreement (the “University Agreement”). Pursuant to the University Agreement, for a term of two years the University agreed to use its reasonable efforts to perform academic research funded by us in connection with the development of point-of-care modular autonomous processing system to generate clinical-grade AVA-011, a RNA-based chimeric antigen receptor (CAR) T-cell therapy candidate with the appointment of Dr. Yen Michael S. Hsu as Principal Investigator. We are in the process of renegotiating this agreement to extend the term through 2023 and complete some of the research contemplated in the original agreement. |
| ● | AVA-Trap™: Avalon’s AVA-Trap™ therapeutic program plans to enter animal model testing followed by expedited clinical studies with the goal of providing an effective therapeutic option to combat COVID-19 and other life-threatening conditions involving cytokine storms. We initiated a sponsored research and co-development project with Massachusetts Institute of Technology (MIT) led by Professor Shuguang Zhang as Principal Investigator in May 2019. Using the unique QTY code protein design platform, six water-soluble variant cytokine receptors have been successfully designed and tested to show binding affinity to the respective cytokines. AVA-TrapTM can potentially generate novel therapeutic targets for cellular therapy, as well as in the field of precision diagnostics. We do not have a timeline for the next steps of this study. |
For the year ended December 31, 2022, we generated rental revenue from our commercial real property in New Jersey, where we are headquartered. Starting in 2023, in addition to the rental, we also plan to generate income through our membership interest in Lab Services MSO.
Corporate and Available Information
We are incorporated in Delaware. Our website is located at http://www.avalon-globocare.com. On our website, investors can obtain, free of charge, a copy of our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, our Code of Conduct and Business Ethics, including disclosure related to any amendments or waivers thereto, other reports and any amendments thereto filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act of 1934, as amended, as soon as reasonably practicable after we file such material electronically with, or furnish it to, the Securities and Exchange Commission, or the SEC. None of the information posted on our website is incorporated by reference into this Annual Report. The SEC also maintains a website at http://www.sec.gov that contains reports, proxy and information statements and other information regarding us and other companies that file materials with the SEC electronically.
China Operations
Due to the winding down of the medical related consulting services segment, in November 2022, we decided to cease all operations in the People’s Republic of China (the “PRC”) with the exception of a small administrative office, in Shanghai. We, through our Nevada Subsidiary Avactis Biosciences Inc., will continue to own Avactis Nanjing Biosciences Ltd., which only owns a patent and is not considered an operating entity. In addition, we reconstituted our board in December 2022 at our annual meeting of stockholders and our directors who were citizens of China did not stand for re-election at our annual meeting. We do not expect nor do we plan that we will further operate in the PRC or generate revenue from PRC operations for the foreseeable future.
2
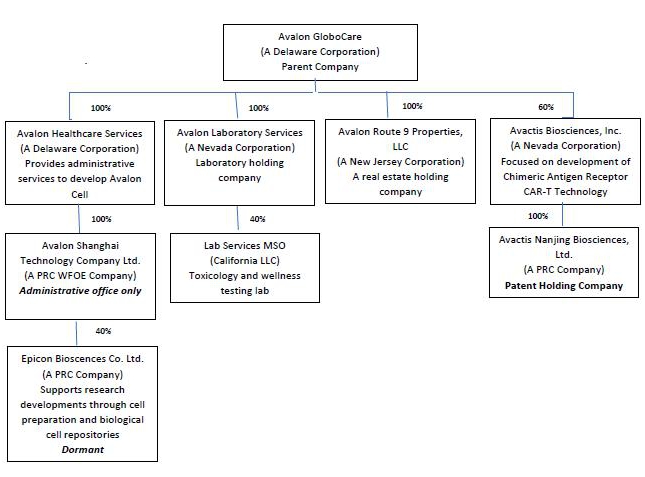
The following diagram illustrates our corporate structure:
Recent Developments
In the fourth quarter of 2022, we conducted a private placement offering for shares of our newly designated Series A Convertible Preferred Stock, stated value $1,000 per share (the “Series A Preferred Stock”). We entered into a securities purchase agreement (the “Securities Purchase Agreement”), with certain accredited investors named therein, including Wenzhao Lu, the chairman of our board of directors, pursuant to which we sold an aggregate of 9,000 shares of our Series A Preferred Stock for the gross proceeds of $9,000,000, which funds were used to pay the cash purchase price in connection with our acquisition of Lab Services.
On February 9, 2023, we entered into and closed an Amended and Restated Membership Interest Purchase Agreement (the “Amended MIPA”), by and among Avalon Laboratory Services, Inc., a wholly-owned subsidiary of us (“Avalon Laboratory Services”), SCBC Holdings LLC, Laboratory Services, the Zoe Family Trust, Bryan Cox and Sarah Cox. The Amended MIPA amended and restated, in its entirety, that certain Membership Interest Purchase Agreement, dated November 7, 2022 (the “Original MIPA”).
Under the Amended MIPA, we acquired from SCBC Holdings LLC through our subsidiary Avalon Laboratory Services, forty percent (40%) of all the issued and outstanding equity interests of Laboratory Services, free and clear of all liens (the “Laboratory Services MSO Acquisition”). As part of the consideration for the Laboratory Services MSO Acquisition, we issued shares of our newly designated Series B Convertible Stock, stated value $1,000 per share (“the Series B Preferred Stock”). Further, Avalon Laboratory Services paid SCBC Holdings LLC $21,000,000 for all the issued and outstanding equity interests of Laboratory Services, which comprised of (i) $9,000,000 in cash, (ii) $11,000,000 pursuant to the issuance of the Series B Preferred Stock, and (iii) a $1,000,000 cash payment on February 9, 2024.
In addition, at any time during the period beginning on the closing date of the Laboratory Services MSO Acquisition and ending on the date nine (9) months after such closing date, Avalon Laboratory Services, or its designated affiliates under the Amended MIPA, may purchase from SCBC Holdings LLC twenty percent (20%) of the total issued and outstanding equity interests of Laboratory Services MSO for the purchase price of (i) $6,000,000 in cash and (ii) the issuance of an additional 4,000 shares of Series B Preferred Stock valued at $4,000,000, in accordance with the terms and conditions set forth in the Amended MIPA.
Sales and Marketing
We seek to develop new business through relationships driven by our senior management, which have extensive contacts throughout the healthcare system. Our senior management will be seeking opportunities for joint ventures, strategic relationships and acquisitions in consulting, biomedical innovations, laboratory, and medical device companies. In addition, through our membership interest in Lab Services, we plan to generate revenue from toxicology and wellness laboratory testing. We also intend to seek opportunities to expand the operations of Lab Services, through acquisition of additional lab companies and through the opening of new lab locations.
3
Consulting Services
Due to the winding down of the medical related consulting services in 2022, the Company decided to cease all operations of Avalon Shanghai and no longer has any material revenues or expenses in Avalon Shanghai.
Markets
Laboratory Services
Through our membership interest in Laboratory Services, we are focused on delivering high quality services related to toxicology and wellness testing. We use fast, accurate, and efficient equipment to provide practitioners with the tools to quickly determine if a patient is following their designated treatment plan. In most instances, we are able to provide a practitioner with qualitative drug class results the same day the sample is received. We provide an extensive chemistry test menu that gives physicians the information to better treat their patients and maintain their overall wellness. The panels that we test for are thyroid panel, comprehensive metabolic panel, kidney profile, liver function tests, and other individual tests.
Cellular Therapy
We focus on the following markets in developing our cellular therapy business:
| ● | Cellular Immunotherapy in Oncology: Regarded as the future of medicine, we believe cell-based technologies and therapeutics will replace pharmaceuticals as a more effective and functional modality in certain unmet medical areas. We are actively engaging in this revolutionary trend and positioning to take a leading role in immune effector cell therapies in the immuno-oncology domai. |
| ● | QTY-Code Protein Design: Novel therapeutic and diagnostic targets development utilizing QTY-code protein design technology with Massachusetts Institute of Technology (MIT) including using the QTY code protein design technology for development of a hemofiltration device to treat Cytokine Storm (aka Cytokine Release Syndrome). QTY-code can be applied to generate water-soluble, antibody-like molecular variants of native membrane-bound receptors, which may expand the repertoire of therapeutic targets in CAR-T cell therapies. |
Revenue
Avalon RT 9 Properties, LLC
In May 2017, we acquired commercial property located in Freehold, New Jersey. This property is now our corporate headquarters and contains several commercial tenants that generate revenue through rental income.
Laboratory Services
On February 9, 2023, we acquired membership interest in Lab Services. We anticipate generating revenue through this membership interest in the areas of toxicology and wellness testing.
Strategic Development
Through our wholly owned subsidiary Lab Services, we plan to embark in a rollup acquisition strategy of small to medium size laboratories accretive to our strategy and complimentary to our membership interest in Lab Services. We also intend to pursue the acquisition and development of healthcare related technologies for cell related diagnostics and therapeutics through acquisition, licensing or joint ventures with major universities and biotech companies. seeking laboratory or medical device acquisitions.
Intellectual Property
Our goal is to obtain, maintain and enforce patent rights for our products, formulations, processes, methods of use and other proprietary technologies, preserve our trade secrets, and operate without infringing on the proprietary rights of other parties, both in the United States and abroad. Our policy is to actively seek to obtain, where appropriate, the broadest intellectual property protection possible for our current product candidates and any future product candidates, proprietary information and proprietary technology through a combination of contractual arrangements and patents, both in the United States and abroad. Even patent protection, however, may not always afford us with complete protection against competitors who seek to circumvent our patents. If we fail to adequately protect or enforce our intellectual property rights or secure rights to patents of others, the value of our intellectual property rights would diminish. To this end, we require all of our employees, consultants, advisors and other contractors to enter into confidentiality agreements that prohibit the disclosure and use of confidential information and, where applicable, require disclosure and assignment to us of the ideas, developments, discoveries and inventions relevant to our technologies and important to our business.
4
Competition
Laboratory Services
While there has been consolidation in the diagnostic information services industry in recent years, the laboratory testing industry is fragmented and highly competitive. We primarily compete with three types of clinical testing providers: commercial clinical laboratories IDN-affiliated laboratories and physician-office laboratories. Our largest commercial clinical laboratory competitors are Quest Diagnostic Laboratories and Laboratory Corporation of America. In addition, we compete with many smaller regional and local commercial clinical laboratories, specialized advanced laboratories and providers of consumer-initiated testing. There also has been a trend among physician practices to establish their own histology laboratory capabilities and/or bring pathologists into their practices, thereby reducing referrals from these practices and increasing the competitive position of these practices.
In addition, we believe that consolidation in the diagnostic information services industry will continue. A significant portion of clinical testing is likely to continue to be performed by independent delivery networks (including hospitals and hospital health systems) (“IDNs”), which generally have affiliations with community clinicians and may have more, or more convenient, locations in a market. As a result, we compete against these affiliated laboratories primarily on the basis of service capability, quality and pricing. In addition, market activity may increase the competitive environment. For example, IDN ownership of physician practices may enhance the ties of the clinicians to IDN-affiliated laboratories, enhancing the competitive position of IDN-affiliated laboratories.
The diagnostic information services industry is faced with changing technology, new product introductions and new service offerings. Competitors may compete using advanced technology, including technology that enables more convenient or cost-effective testing. Digital pathology, still in an emerging state, is an example of this. Competitors also may compete on the basis of new service offerings. Competitors also may offer testing to be performed outside of a commercial clinical laboratory, such as (1) point-of-care testing that can be performed by physicians in their offices; (2) testing that can be performed by IDNs in their own laboratories; and (3) home testing that can be carried out without requiring the services of outside providers.
Clinical
The development and commercialization of new drug products is highly competitive. We expect that we will face significant competition from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide with respect to our product candidates that we may seek to develop or commercialize in the future. Specifically, due to the large unmet medical need, global demographics and relatively attractive reimbursement dynamics, the markets in which we are seeking to develop products are fiercely competitive and there are a number of large pharmaceutical and biotechnology companies that currently market and sell products or are pursuing the development of product candidates similar to ours. Our competitors may succeed in developing, acquiring or licensing technologies and drug products that are more effective, have fewer or more tolerable side effects or are less costly than any product candidates that we are currently developing or that we may develop, which could render our product candidates obsolete and noncompetitive.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. Our competitors also may obtain FDA or other marketing approval for their products before we are able to obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market.
5
General
Many of our existing and potential future competitors have significantly greater financial resources and expertise in lab services and operations, research and development, manufacturing, preclinical testing, conducting clinical studies, obtaining marketing approvals and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller, or early stage, companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical study sites and patient registration for clinical studies, as well as in acquiring technologies complementary to, or necessary for, our programs.
We expect that our ability to compete effectively will depend upon our ability to:
| ● | successfully operate and expand our lab services and locations; |
| ● | successfully and rapidly complete adequate and well-controlled clinical studies that demonstrate statistically significant safety and efficacy and to obtain all requisite regulatory approvals in a cost-effective manner; |
| ● | maintain a proprietary position for our manufacturing processes and other technology; |
| ● | produce our products in accordance with FDA and international regulatory guidelines; |
| ● | attract and retain key personnel; and |
| ● | build or access an adequate sales and marketing infrastructure for any approved products. |
Failure to do one or more of these activities could have an adverse effect on our business, financial condition or results of operations.
Avalon RT 9 Properties LLC
Our executive commercial building in Freehold, New Jersey is located on a major highway and is one of the largest buildings in the surrounding areas. It is centrally located and maintains high occupancy. There are other commercial properties in the vicinity that offer similar amenities. However, premier executive offices are limited and as such we expect to continue to maintain high occupancy in the near term.
Employees
As of March 30, 2023, we employed six employees, five of which are full time employees. None of our employees are represented by a collective bargaining arrangement.
Government Regulation
Overview
The healthcare industry in the U.S. is highly regulated and subject to changing political, legislative, regulatory, and other influences. Further, the healthcare industry is currently undergoing rapid change. We are uncertain how, when or in what context these new changes will be adopted or implemented. These new regulations could create unexpected liabilities for us, could cause us or our members to incur additional costs and could restrict our or our clients’ operations. Many of the laws are complex and their application to us, our clients, or the specific services and relationships we have with our members are not always clear. Our failure to anticipate accurately the application of these laws and regulations, or our other failure to comply, could create liability for us, result in adverse publicity, and otherwise negatively affect our business.
6
Holding Foreign Companies Accountable Act Compliance
The Holding Foreign Companies Accountable Act, or the HFCA Act, was enacted on December 18, 2020. According to the HFCA Act, if the SEC determines that Avalon has filed audit reports issued by a registered public accounting firm that has not been subject to inspection by the PCAOB for three consecutive years beginning in 2021, the SEC will prohibit Avalon’s securities from being traded on a national securities exchange or in the over-the-counter trading market in the United States.
On December 16, 2021, the PCAOB issued a Determination Report which reported that the PCAOB is unable to inspect or investigate completely registered public accounting firms headquartered in: (1) mainland China of the People’s Republic of China, because of a position taken by one or more authorities in mainland China; and (2) Hong Kong, a Special Administrative Region of the PRC, because of a position taken by one or more authorities in Hong Kong.
Avalon’s auditor is Marcum LLP (“Marcum”), based in New York, New York. Marcum is registered with the PCAOB and is subject to laws in the United States pursuant to which the PCAOB conducts regular inspections to assess their compliance with the applicable professional standards. Since Marcum is located in the United States, the PCAOB has been able to conduct inspections of Marcum. In addition, Marcum is not among the PCAOB registered public accounting firms registered in mainland China or Hong Kong that are subject to PCAOB’s determination on December 16, 2021.
Although the audit reports of Avalon are prepared by U.S. auditors that are subject to inspection by the PCAOB, the PCAOB is currently unable to conduct inspections over the audit work of Avalon’s independent registered public accounting firms with respect to Avalon’s operations in mainland China without the approval of certain Chinese authorities. Also, there is no guarantee that future audit reports will be prepared by auditors that are completely inspected by the PCAOB and, as such, future investors may be deprived of such inspections, which could result in limitations or restrictions to Avalon’s access of the U.S. capital markets.
Inspections of certain other firms that the PCAOB has conducted outside of China have identified deficiencies in those firms’ audit procedures and quality control procedures, which may be addressed as part of the inspection process to improve future audit quality. However, the PCAOB is currently unable to inspect an auditor’s audit work related to a company’s operations in China where such documentation of the audit work is located in China. As a result, Avalon’s investors may be deprived of the benefits of the PCAOB’s oversight of auditors that are located in China through such inspections.
On March 24, 2021, the SEC adopted interim final rules relating to the implementation of certain disclosure and documentation requirements of the HFCA Act. Avalon will be required to comply with these rules if the SEC identifies us as having a “non-inspection” year under a process to be subsequently established by the SEC. The SEC is assessing how to implement other requirements of the HFCA Act, including the listing and trading prohibition requirements described above.
On June 22, 2021, the U.S. Senate passed a bill which, if passed by the U.S. House of Representatives and signed into law, would reduce the number of consecutive non-inspection years required for triggering the prohibitions under the HFCA Act from three years to two, which would shorten the timeframe before Avalon’s share may be delisted and before the trading in Avalon’s shares is prohibited.
On November 5, 2021, the SEC approved Rule 6100 adopted by the PCAOB to determine its inability to inspect or investigate registered firms completely under the HFCA Act. This rule establishes the framework for the PCAOB to make these required determinations. The trading in Avalon’s securities may be prohibited under the HFCA Act if the PCAOB subsequently determines Avalon’s audit work is performed by auditors that the PCAOB is unable to inspect or investigate completely pursuant to Rule 6100, and as a result, U.S. national securities exchanges, such as Nasdaq, may determine to delist Avalon’s securities. Such a delisting would likely cause the value of such securities to significantly decline or become worthless.
7
The SEC may propose additional regulatory or legislative requirements or guidance that could impact us if our auditor is not subject to PCAOB inspection. For example, on August 6, 2020, the President’s Working Group on Financial Markets, or the PWG, issued the Report on Protecting United States Investors from Significant Risks from Chinese Companies to the then President of the United States. This report recommended the SEC implement five recommendations to address companies from jurisdictions that do not provide the PCAOB with sufficient access to fulfil its statutory mandate. Some of the concepts of these recommendations were implemented with the enactment of the HFCA Act. However, some of the recommendations were more stringent than the HFCA Act. For example, if a company was not subject to PCAOB inspection, the report recommended that the transition period before a company would be delisted would end on January 1, 2022.
The SEC has announced that the SEC staff is preparing a consolidated proposal for the rules regarding the implementation of the HFCA Act and to address the recommendations in the PWG report. It is unclear when the SEC will complete its rulemaking and when such rules will become effective and what, if any, of the PWG recommendations will be adopted. The implications of this possible regulation in addition to the requirements of the HFCA Act are uncertain. Although Avalon is currently not subject to the HFCA Act, any uncertainty of its applicability to Avalon, for example if Avalon switched to using a PRC-based auditing firm, could cause the market price of Avalon’s securities to be materially and adversely affected and could cause Avalon’s securities to be delisted or prohibited from being traded “over-the-counter”. If Avalon’s securities are unable to be listed on another securities exchange, such a delisting would substantially impair your ability to sell or purchase Avalon’s securities when you wish to do so, and the risk and uncertainty associated with a potential delisting would have a negative impact on the price of Avalon’s securities. See “Risk Factors— Trading in Avalon’s securities may be restricted under the Holding Foreign Companies Accountable Act if the PCAOB determines that it cannot inspect or fully investigate Avalon’s auditors, and as a result, U.S. national securities exchanges, such as Nasdaq, may determine to delist Avalon’s securities.
Drug Approval Process
The research, development, testing, manufacture, labeling, promotion, advertising, distribution and marketing, among other things, of our product candidates are extensively regulated by governmental authorities in the United States and other countries. In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, or the FDCA, and its implementing regulations. Failure to comply with the applicable U.S. requirements may subject us to administrative or judicial sanctions, such as the FDA’s refusal to approve a pending new drug application, or NDA, or a pending biologics license application, or BLA, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions and/or criminal prosecution.
Pharmaceutical products such as ours may not be commercially marketed without prior approval from the FDA and comparable regulatory agencies in other countries. In the United States, the process to receiving such approval is long, expensive and risky, and includes the following steps:
| ● | pre-clinical laboratory tests, animal studies, and formulation studies; |
| ● | submission to the FDA of an IND for human clinical testing, which must become effective before human clinical trials may begin; |
| ● | adequate and well-controlled human clinical trials to establish the safety and efficacy of the drug for each indication; |
| ● | submission to the FDA of an NDA or BLA; |
| ● | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the drug is produced to assess compliance with current good manufacturing practices, or cGMPs; |
| ● | a potential FDA audit of the preclinical and clinical trial sites that generated the data in support of the NDA or BLA; |
| ● | the ability to obtain clearance or approval of companion diagnostic tests, if required, on a timely basis, or at all; and |
| ● | FDA review and approval of the NDA or BLA. |
8
Regulation by U.S. and foreign governmental authorities is a significant factor affecting our ability to commercialize any of our products, as well as the timing of such commercialization and our ongoing research and development activities. The commercialization of drug products requires regulatory approval by governmental agencies prior to commercialization. Various laws and regulations govern or influence the research and development, non-clinical and clinical testing, manufacturing, processing, packing, validation, safety, labeling, storage, record keeping, registration, listing, distribution, advertising, sale, marketing and post-marketing commitments of our products. The lengthy process of seeking these approvals, and the subsequent compliance with applicable laws and regulations, require expending substantial resources.
The results of pre-clinical testing, which include laboratory evaluation of product chemistry and formulation, animal studies to assess the potential safety and efficacy of the product and its formulations, details concerning the drug manufacturing process and its controls, and a proposed clinical trial protocol and other information must be submitted to the FDA as part of an IND that must be reviewed and become effective before clinical testing can begin. The study protocol and informed consent information for patients in clinical trials must also be submitted to an independent Institutional Review Board, or IRB, for approval covering each institution at which the clinical trial will be conducted. Once a sponsor submits an IND, the sponsor must wait 30 calendar days before initiating any clinical trials. If the FDA has comments or questions within this 30-day period, the issue(s) must be resolved to the satisfaction of the FDA before clinical trials can begin. In addition, the FDA, an IRB or the company may impose a clinical hold on ongoing clinical trials due to safety concerns. If the FDA imposes a clinical hold, clinical trials can only proceed under terms authorized by the FDA. Our pre-clinical and clinical studies must conform to the FDA’s Good Laboratory Practice, or GLP, and Good Clinical Practice, or GCP, requirements, respectively, which are designed to ensure the quality and integrity of submitted data and protect the rights and well-being of study patients. Information for certain clinical trials also must be publicly disclosed within certain time limits on the clinical trial registry and results databank maintained by the NIH.
Typically, clinical testing involves a three-phase process; however, the phases may overlap or be combined:
| ● | Phase I clinical trials typically are conducted in a small number of volunteers or patients to assess the early tolerability and safety profile, and the pattern of drug absorption, distribution and metabolism; |
| ● | Phase II clinical trials typically are conducted in a limited patient population with a specific disease in order to assess appropriate dosages and dose regimens, expand evidence of the safety profile and evaluate preliminary efficacy; and |
| ● | Phase III clinical trials typically are larger scale, multicenter, well-controlled trials conducted on patients with a specific disease to generate enough data to statistically evaluate the efficacy and safety of the product, to establish the overall benefit-risk relationship of the drug and to provide adequate information for the registration of the drug. |
A therapeutic product candidate being studied in clinical trials may be made available for treatment of individual patients, in certain circumstances. Pursuant to the 21st Century Cures Act (Cures Act), which was signed into law in December 2016. The manufacturer of an investigational product for a serious disease or condition is required to make available, such as by posting on its website, its policy on evaluating and responding to requests for individual patient access to such investigational product.
The results of the pre-clinical and clinical testing, chemistry, manufacturing and control information, proposed labeling and other information are then submitted to the FDA in the form of either an NDA or BLA for review and potential approval to begin commercial sales. In responding to an NDA or BLA, the FDA may grant marketing approval, request additional information in a Complete Response Letter, or CRL, or deny the approval if it determines that the NDA or BLA does not provide an adequate basis for approval. A CRL generally contains a statement of specific conditions that must be met in order to secure final approval of an NDA or BLA and may require additional testing. If and when those conditions have been met to the FDA’s satisfaction, the FDA will typically issue an approval letter, which authorizes commercial marketing of the product with specific prescribing information for specific indications, and sometimes with specified post-marketing commitments and/or distribution and use restrictions imposed under a Risk Evaluation and Mitigation Strategy program. Any approval required from the FDA might not be obtained on a timely basis, if at all.
9
Among the conditions for an NDA or BLA approval is the requirement that the manufacturing operations conform on an ongoing basis with cGMPs. In complying with cGMPs, we must expend time, money and effort in the areas of training, production and quality control within our own organization and at our contract manufacturing facilities. A successful inspection of the manufacturing facility by the FDA is usually a prerequisite for final approval of a pharmaceutical product. Following approval of the NDA or BLA, we and our manufacturers will remain subject to periodic inspections by the FDA to assess compliance with cGMPs requirements and the conditions of approval. We will also face similar inspections coordinated by foreign regulatory authorities.
Disclosure of Clinical Trial Information
Sponsors of certain clinical trials of FDA-regulated products are required to register and disclose certain clinical trial information. Information related to the product, patient population, phase of investigation, trial sites and investigators, and other aspects of the clinical trial are then made public as part of the registration. Sponsors are also obligated to disclose the results of their clinical trials after completion. Disclosure of the results of these trials can be delayed in certain circumstances for up to two years after the date of completion of the trial. Competitors may use this publicly available information to gain knowledge regarding the progress of development programs.
Expedited Development and Review Programs
The FDA has a Fast Track program that is intended to expedite or facilitate the process for reviewing new drugs and biological products that meet certain criteria. Specifically, new drugs and biological products are eligible for Fast Track designation if they are intended to treat a serious or life-threatening condition and demonstrate the potential to address unmet medical needs for the condition. Fast Track designation applies to the combination of the product and the specific indication for which it is being studied. The sponsor of a new drug or biologic may request the FDA to designate the drug or biologic as a Fast Track product at any time during the clinical development of the product. Unique to a Fast Track product, the FDA may consider for review sections of the marketing application on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the application, the FDA agrees to accept sections of the application and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the application.
Any product submitted to the FDA for marketing, including under a Fast Track program, may be eligible for other types of FDA programs intended to expedite development and review, such as priority review and accelerated approval. Under the Breakthrough Therapy program, products intended to treat a serious or life-threatening disease or condition may be eligible for the benefits of the Fast Track program when preliminary clinical evidence demonstrates that such product may have substantial improvement on one or more clinically significant endpoints over existing therapies. Additionally, FDA will seek to ensure the sponsor of a breakthrough therapy product receives timely advice and interactive communications to help the sponsor design and conduct a development program as efficiently as possible. Any product is eligible for priority review if it has the potential to provide safe and effective therapy where no satisfactory alternative therapy exists or a significant improvement in the treatment, diagnosis or prevention of a disease compared to marketed products. The FDA will attempt to direct additional resources to the evaluation of an application for a new drug or biological product designated for priority review in an effort to facilitate the review. Additionally, a product may be eligible for accelerated approval. Drug or biological products studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may receive accelerated approval, which means that they may be approved on the basis of adequate and well-controlled clinical studies establishing that the product has an effect on a surrogate endpoint that is reasonably likely to predict a clinical benefit, or on the basis of an effect on a clinical endpoint other than survival or irreversible morbidity. As a condition of approval, the FDA may require that a sponsor of a drug or biological product receiving accelerated approval perform adequate and well-controlled post-marketing clinical studies. In addition, the FDA currently requires as a condition for accelerated approval the pre-approval of promotional materials, which could adversely impact the timing of the commercial launch of the product. Fast Track designation, Breakthrough Therapy designation, priority review and accelerated approval do not change the standards for approval but may expedite the development or approval process.
10
Regenerative Medicine Advanced Therapies (RMAT) Designation
The FDA has established a Regenerative Medicine Advanced Therapy, or RMAT, designation as part of its implementation of the 21st Century Cures Act, or Cures Act. The RMAT designation program is intended to fulfill the Cures Act requirement that the FDA facilitate an efficient development program for, and expedite review of, any drug that meets the following criteria: (1) it qualifies as a RMAT, which is defined as a cell therapy, therapeutic tissue engineering product, human cell and tissue product, or any combination product using such therapies or products, with limited exceptions; (2) it is intended to treat, modify, reverse, or cure a serious or life-threatening disease or condition; and (3) preliminary clinical evidence indicates that the drug has the potential to address unmet medical needs for such a disease or condition. Like breakthrough therapy designation, RMAT designation provides potential benefits that include more frequent meetings with FDA to discuss the development plan for the product candidate, and eligibility for rolling review and priority review. Products granted RMAT designation may also be eligible for accelerated approval on the basis of a surrogate or intermediate endpoint reasonably likely to predict long-term clinical benefit, or reliance upon data obtained from a meaningful number of sites, including through expansion to additional sites. RMAT-designated products that receive accelerated approval may, as appropriate, fulfill their post-approval requirements through the submission of clinical evidence, clinical studies, patient registries, or other sources of real world evidence (such as electronic health records); through the collection of larger confirmatory data sets; or via post-approval monitoring of all patients treated with such therapy prior to approval of the therapy.
Post-Approval Requirements
Oftentimes, even after a drug has been approved by the FDA for sale, the FDA may require that certain post-approval requirements be satisfied, including the conduct of additional clinical studies. If such post-approval requirements are not satisfied, the FDA may withdraw its approval of the drug. In addition, holders of an approved NDA or BLA are required to report certain adverse reactions to the FDA, comply with certain requirements concerning advertising and promotional labeling for their products, and continue to have quality control and manufacturing procedures conform to cGMPs after approval. The FDA periodically inspects the sponsor’s records related to safety reporting and/or manufacturing facilities; this latter effort includes assessment of compliance with cGMPs. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMPs compliance.
Other Healthcare Fraud and Abuse Laws
In the U.S., our activities are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including but not limited to, the Centers for Medicare and Medicaid Services, or CMS, other divisions of the U.S. Department of Health and Human Services (such as the Office of Inspector General and the Health Resources and Service Administration), the U.S. Department of Justice, or the DOJ, and individual U.S. Attorney offices within the DOJ, and state and local governments. For example, sales, marketing and scientific/educational grant programs may have to comply with the anti-fraud and abuse provisions of the Social Security Act, the false claims laws, the privacy and security provisions of the Health Insurance Portability and Accountability Act, or HIPAA, and similar state laws, each as amended, as applicable.
The federal Anti-Kickback Statute prohibits, among other things, any person or entity from knowingly and willfully offering, paying, soliciting or receiving any remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce or in return for purchasing, leasing, ordering or arranging for the purchase, lease or order of any item or service reimbursable, in whole or in part, under Medicare, Medicaid or other federal healthcare programs. The term remuneration has been interpreted broadly to include anything of value. The Anti-Kickback Statute has been interpreted to apply to arrangements between therapeutic product manufacturers on one hand and prescribers, purchasers, and formulary managers on the other. There are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution. The exceptions and safe harbors are drawn narrowly and practices that involve remuneration that may be alleged to be intended to induce prescribing, purchasing or recommending may be subject to scrutiny if they do not qualify for an exception or safe harbor. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the Anti-Kickback Statute. Instead, the legality of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all of its facts and circumstances. Additionally, the intent standard under the Anti-Kickback Statute was amended by the ACA to a stricter standard such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. In addition, the ACA codified case law that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act, or FCA.
11
The federal false claims and civil monetary penalty laws, including the FCA, which imposes significant penalties and can be enforced by private citizens through civil qui tam actions, prohibit any person or entity from, among other things, knowingly presenting, or causing to be presented, a false or fraudulent claim for payment to, or approval by, the federal healthcare programs, including Medicare and Medicaid, or knowingly making, using, or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government. A claim includes “any request or demand” for money or property presented to the U.S. government. For instance, historically, pharmaceutical and other healthcare companies have been prosecuted under these laws for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. Other companies have been prosecuted for causing false claims to be submitted because of the companies’ marketing of the product for unapproved, off-label, and thus generally non-reimbursable, uses.
HIPAA created additional federal criminal statutes that prohibit, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud or to obtain, by means of false or fraudulent pretenses, representations or promises, any money or property owned by, or under the control or custody of, any healthcare benefit program, including private third-party payors, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up by trick, scheme or device, a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Like the Anti-Kickback Statute, the ACA amended the intent standard for certain healthcare fraud statutes under HIPAA such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
Many states have similar, and typically more prohibitive, fraud and abuse statutes or regulations that apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. Additionally, to the extent that our product candidates may in the future be sold in a foreign country, we may be subject to similar foreign laws.
We may be subject to data privacy and security regulations by both the federal government and the states in which we conduct our business. HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and its implementing regulations, imposes requirements relating to the privacy, security and transmission of individually identifiable health information. Among other things, HITECH makes HIPAA’s privacy and security standards directly applicable to business associates, independent contractors, or agents of covered entities that receive or obtain protected health information in connection with providing a service on behalf of a covered entity. HITECH also created four new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce HIPAA and seek attorneys’ fees and costs associated with pursuing federal civil actions. In addition, many state laws govern the privacy and security of health information in specified circumstances, many of which differ from each other in significant ways, are often not pre-empted by HIPAA, and may have a more prohibitive effect than HIPAA, thus complicating compliance efforts.
We expect our product, after approval, may be eligible for coverage under Medicare, the federal health care program that provides health care benefits to the aged and disabled, and covers outpatient services and supplies, including certain pharmaceutical products, that are medically necessary to treat a beneficiary’s health condition. In addition, the product may be covered and reimbursed under other government programs, such as Medicaid and the 340B Drug Pricing Program. The Medicaid Drug Rebate Program requires pharmaceutical manufacturers to enter into and have in effect a national rebate agreement with the Secretary of the Department of Health and Human Services as a condition for states to receive federal matching funds for the manufacturer’s outpatient drugs furnished to Medicaid patients. Under the 340B Drug Pricing Program, the manufacturer must extend discounts to entities that participate in the program. As part of the requirements to participate in certain government programs, many pharmaceutical manufacturers must calculate and report certain price reporting metrics to the government, such as average manufacturer price, or AMP, and best price. Penalties may apply in some cases when such metrics are not submitted accurately and timely.
12
Additionally, the federal Physician Payments Sunshine Act, or the Sunshine Act, within the ACA, and its implementing regulations, require that certain manufacturers of drugs, devices, biological and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) report annually to CMS information related to certain payments or other transfers of value made or distributed to physicians and teaching hospitals, or to entities or individuals at the request of, or designated on behalf of, the physicians and teaching hospitals and to report annually certain ownership and investment interests held by physicians and their immediate family members. Failure to report accurately could result in penalties. In addition, many states also govern the reporting of payments or other transfers of value, many of which differ from each other in significant ways, are often not pre-empted, and may have a more prohibitive effect than the Sunshine Act, thus further complicating compliance efforts.
New Legislation and Regulations
From time to time, legislation is drafted, introduced and passed in Congress that could significantly change the statutory provisions governing the testing, approval, manufacturing and marketing of products regulated by the FDA. In addition to new legislation, FDA regulations and policies are often revised or interpreted by the agency in ways that may significantly affect our business and our products. It is impossible to predict whether further legislative changes will be enacted or whether FDA regulations, guidance, policies or interpretations will be changed or what the effect of such changes, if any, may be.
ITEM 1A. RISK FACTORS
You should carefully consider the following material risk factors as well as all other information set forth or referred to in this report before purchasing shares of our common stock. Investing in our common stock involves a high degree of risk. We may not be successful in preventing the material adverse effects that any of the following risks and uncertainties may cause. These potential risks and uncertainties may not be a complete list of the risks and uncertainties facing us. There may be additional risks and uncertainties that we are presently unaware of, or presently consider immaterial, that may become material in the future and have a material adverse effect on us. You could lose all or a significant portion of your investment due to any of these risks and uncertainties.
Summary of Risk Factors
Our business is subject to numerous risks and uncertainties that you should consider before investing in our company, as fully described below. The principal factors and uncertainties that make investing in our company risky include, among others:
General Operating and Business Risks
| ● | Our limited operating history makes it difficult for us to evaluate our future business prospects and make decisions based on those estimates of our future performance. | |
| ● | Our results of operations have not resulted in profitability and we may not be able to achieve profitability going forward. | |
| ● | There is substantial doubt about our ability to continue as a going concern, which will affect our ability to obtain future financing and may require us to curtail our operations. | |
| ● | Our cash will only fund our operations for a limited time and we will need to raise additional capital in order to support our development. |
| ● | The Laboratory Services MSO Acquisition will result in organizational changes that could create significant growth for our business. If we fail to effectively manage this growth and adapt our business structure in a manner that preserves our reputation, then our business, financial condition and results of operations could be harmed. |
| ● | We must effectively manage the growth of our operations, or our company will suffer. |
13
| ● | Our prospects will suffer if we are not able to hire, train, motivate, manage, and retain a significant number of highly skilled employees. |
| ● | Potential liability claims may adversely affect our business. |
| ● | In accordance with our strategic development policy, we may invest in companies for strategic reasons and may not realize a return on our investments. |
| ● | Obtaining and maintaining patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and any patent protection we may obtain in the future could be reduced or eliminated for non-compliance with these requirements. |
| ● | It is difficult and costly to protect our proprietary rights, and we may not be able to ensure their protection. If we fail to protect or enforce our intellectual property rights adequately or secure rights to patents of others, the value of our intellectual property rights would diminish. |
| ● | If any of our trade secrets, know-how or other proprietary information is disclosed, the value of our trade secrets, know-how and other proprietary rights would be significantly impaired and our business and competitive position would suffer. |
Risk Factors Related to our Laboratory Services Business
| ● | Continued changes in healthcare reimbursement models and products, changes in government payment and reimbursement systems, or changes in payer mix could have a material adverse effect on our revenues, profitability and cash flow. | |
| ● | The clinical testing business is highly competitive, and if we fail to provide an appropriately priced level of service or otherwise fail to compete effectively it could have a material adverse effect on our revenues and profitability. | |
| ● | Failure to obtain and retain new customers, the loss of existing customers or material contracts, or a reduction in services or tests ordered or specimens submitted by existing customers, or the inability to retain existing and/or create new relationships with health systems could impact our ability to successfully grow our business. |
| ● | Discontinuation or recalls of existing testing products; failure to develop or acquire licenses for new or improved testing technologies; or our customers using new technologies to perform their own tests could adversely affect our business. | |
| ● | Continued and increased consolidation of pharmaceutical, biotechnology and medical device companies, health systems, physicians and other customers could adversely affect our business. |
Risk Factors Related to Clinical and Commercialization Activity
| ● | We may not be able to file INDs to commence additional clinical trials on the timelines we expect, and even if we are able to do so, the FDA may not permit us to proceed. | |
| ● | We have limited experience in conducting clinical trials. | |
| ● | Delays in the commencement, enrollment, and completion of clinical testing could result in increased costs to us and delay or limit our ability to obtain regulatory approval for our product candidates. | |
| ● | As the results of earlier pre-clinical studies or clinical trials are not necessarily predictive of future results, any product candidate we advance into clinical trials may not have favorable results in later clinical trials or receive regulatory approval. | |
| ● | Even if our product candidates receive regulatory approval, we may still face future development and regulatory difficulties. | |
| ● | Any cell based therapies we develop may become subject to unfavorable pricing regulations, third party coverage and reimbursement practices or healthcare reform initiatives, thereby harming our business. |
14
Risks Related to Our Securities
| ● | Our officers, directors and principal stockholders own a significant percentage of our capital stock and will be able to exert significant control over matters that are subject to stockholder approval. |
| ● | If we are unable to maintain listing of our securities on the Nasdaq Capital Market or another reputable stock exchange, it may be more difficult for our stockholders to sell their securities. | |
| ● | The price of our common stock may be volatile and fluctuate substantially, which could result in substantial losses for our stockholders. | |
| ● | You may experience dilution of your ownership interests because of the future issuance of additional shares of our common or preferred stock or other securities that are convertible into or exercisable for our common or preferred stock. |
General Operating and Business Risks
Our limited operating history makes it difficult for us to evaluate our future business prospects and make decisions based on those estimates of our future performance.
We did not begin operations of our business through AHS until May 2015. We have a limited operating history and limited revenue. As a consequence, it is difficult, if not impossible, to forecast our future results based upon our historical data. Reliance on the historical results may not be representative of the results we will achieve, particularly in our combined form. Because of the uncertainties related to our lack of historical operations, we may be hindered in our ability to anticipate and timely adapt to increases or decreases in revenues or expenses. If we make poor budgetary decisions as a result of unreliable historical data, we could be less profitable or incur losses, which may result in a decline in our stock price.
Our results of operations have not resulted in profitability and we may not be able to achieve profitability going forward.
We incurred net losses amounting to $11,930,847 and $9,090,499 for the years ended December 31, 2022 and 2021, respectively. As of December 31, 2022, we had an accumulated deficit of approximately $63.1 million. If we incur additional significant losses, our stock price may decline, perhaps significantly. Our management is developing plans to achieve profitability. Our business plan is speculative and unproven. There is no assurance that we will be successful in executing our business plan or that even if we successfully implement our business plan, that we will be able to curtail our losses now or in the future. Further, as we are a new enterprise, we expect that net losses will continue.
There is substantial doubt about our ability to continue as a going concern, which will affect our ability to obtain future financing and may require us to curtail our operations.
Our financial statements as of December 31, 2022 were prepared under the assumption that we will continue as a going concern. The independent registered public accounting firm that audited our 2022 financial statements, in their report, included an explanatory paragraph referring to our recurring losses since inception and expressing management’s assessment and conclusion that there is substantial doubt in our ability to continue as a going concern. Our financial statements do not include any adjustments that might result from the outcome of this uncertainty. Our ability to continue as a going concern depends on our ability to obtain additional equity or debt financing, attain further operating efficiencies, reduce expenditures, and, ultimately, to generate revenue. We cannot assure you, however, that we will be able to achieve any of the foregoing. See Note 2 to our Consolidated Financial Statements for further details.
15
Our cash will only fund our operations for a limited time and we will need to raise additional capital in order to support our development.
We are currently operating at a loss and expect our operating costs will increase significantly as we continue to grow our operations. The independent registered public accounting firm that audited our 2022 financial statements, in their report, included an explanatory paragraph referring to our recurring losses since inception and expressing management’s assessment and conclusion that there is substantial doubt in our ability to continue as a going concern. At December 31, 2022, we had cash of approximately $2.0 million. We will need to raise additional capital or generate substantial revenue in order to support our development and commercialization efforts.
If our available cash balances are insufficient to satisfy our liquidity requirements, including due to risks described herein, we may seek to raise additional capital through equity offerings, debt financings, collaborations or licensing arrangements. We will need to raise additional capital, and we may also consider raising additional capital in the future to expand our business, to pursue strategic investments, to take advantage of financing opportunities, or for other reasons, including to:
| ● | fund development and expansion of our operations; |
| ● | acquire, license or invest in technologies and additional laboratories; |
| ● | acquire or invest in complementary businesses or assets; and |
| ● | finance capital expenditures and general and administrative expenses. |
Our present and future funding requirements will depend on many factors, including:
| ● | our revenue growth rate and ability to generate cash flows from operating activities; |
| ● | our sales and marketing and research and development activities; and |
| ● | changes in regulatory oversight applicable to our products and services. |
Other than our debt facility with our chairman, we have no arrangements or credit facilities in place as a source of funds, and there can be no assurance that we will be able to raise sufficient additional capital on acceptable terms, or at all, and if we are not successful in raising additional capital, we may not be able to continue as a going concern. We may seek additional capital through a combination of private and public equity offerings, debt financings and strategic collaborations. Debt financing, if obtained, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, that could increase our expenses and require that our assets secure such debt. Equity financing, if obtained, could result in dilution to our then existing stockholders and/or require such stockholders to waive certain rights and preferences. If such financing is not available on satisfactory terms, or is not available at all, we may be required to delay, scale back or eliminate the development of business opportunities and our operations and financial condition may be materially adversely affected. We can provide no assurances that any additional sources of financing will be available to us on favorable terms, if at all. Future capital raises may dilute our existing stockholders’ ownership and/or have other adverse effects on our operations.
If we raise additional capital by issuing equity securities, our existing stockholders’ percentage ownership will be reduced and these stockholders may experience substantial dilution.
If we raise additional funds by issuing debt securities, these debt securities would have rights senior to those of our Common Stock and the terms of the debt securities issued could impose significant restrictions on our operations, including liens on our assets. If we raise additional funds through collaborations and licensing arrangements, we may be required to relinquish some rights to our technologies or products, or to grant licenses on terms that are not favorable to us.
We have significant outstanding debt obligations and servicing these debt obligations will require a significant amount of capital, and our business may not be able to pay our substantial debt.
As of December 31, 2022, we had $4.8 million of outstanding indebtedness. In order to service this indebtedness and any additional indebtedness we may incur in the future, we will need to generate cash from our operating activities. Our ability to generate cash is subject, in part, to our ability to successfully execute our business strategy, as well as general economic, financial, competitive, regulatory and other factors beyond our control. If we are unable to generate sufficient cash to repay our debt obligations when they become due and payable, either when they mature, or in the event of a default, we may not be able to obtain additional debt or equity financing on favorable terms, if at all, which may negatively impact our business operations and financial condition.
16
If we breach any of the undertakings or default on any of our obligations under our agreements with our lenders, our outstanding indebtedness could become immediately due and payable, which would harm our business, financial condition and results of operations and could require us to reduce or cease operations. If our indebtedness were to be accelerated, there can be no assurance that our assets would be sufficient to repay in full that indebtedness.
Our business is subject to risks arising from epidemic diseases, such as the outbreak of the COVID-19 illness.
The Coronavirus Disease 2019, or COVID-19, pandemic which has been declared by the World Health Organization to be a “public health emergency of international concern,” spread across the globe and impacted worldwide economic activity. Although several vaccines have been developed, a public health epidemic, including COVID-19, poses the risk that we or our employees, contractors, suppliers, and other partners may be prevented from conducting business activities for an indefinite period of time, including due to shutdowns that may be requested or mandated by governmental authorities. While it is not possible at this time to estimate the full impact that future pandemics, including COVID-19, could have on our business, the continued spread of COVID-19 and the measures taken by the governments of countries affected could disrupt the supply chain and adversely impact our business, financial condition or results of operations. Future pandemics, including COVID-19, and mitigation measures may also have an adverse impact on global economic conditions which could have an adverse effect on our business and financial condition. The extent to which these pandemics impact our results will depend on future developments that are highly uncertain and cannot be predicted, including new information that may emerge concerning the severity of the virus and the actions to contain its impact.
We depend upon key personnel and need additional personnel.
Our success depends on the continuing services of Wenzhao Lu, our Chairman of the Board, and David Jin, Meng Li and Luisa Ingargiola, our executive officers. The loss of Mr. Lu, Dr. Jin, Ms. Li or Ms. Ingargiola could have a material and adverse effect on our business operations. Additionally, the success of our operations will largely depend upon our ability to successfully attract and maintain competent and qualified key management personnel. As with any company with limited resources, there can be no guaranty that we will be able to attract such individuals or that the presence of such individuals will necessarily translate into profitability for us. Our inability to attract and retain key personnel may materially and adversely affect our business operations. The supply of qualified technical, professional, managerial and other personnel, including lab medical directors and lab operations managers, is currently constrained; competition for qualified employees, even across different industries, is intense, including as individuals leave the job market. We may lose, or fail to attract and retain, key management personnel, or qualified skilled technical, professional or other employees. The same is true for patient-facing staff with specialized training required to perform activities related to specimen collection. In the future, if competition for the services of these professionals increases, we may not be able to continue to attract and retain individuals in its markets. Changes in key management, or the ability to attract and retain qualified personnel, as a result of increased competition for talent, wage growth, or other market factors, could lead to strategic and operational challenges and uncertainties, distractions of management from other key initiatives, and inefficiencies and increased costs, any of which could adversely affect our business, financial condition, results of operations, and cash flows.
The Laboratory Services MSO Acquisition will result in organizational changes that could create significant growth for our business. If we fail to effectively manage this growth and adapt our business structure in a manner that preserves our reputation, then our business, financial condition and results of operations could be harmed.
On February 9, 2023, we acquired 40% of all the issued and outstanding equity interests of Laboratory Services MSO. The Laboratory Services MSO Acquisition has resulted in significant growth in our operations. We have incurred and will continue to incur significant expenditures and the allocation of management time to assimilate Laboratory Services MSO in a manner that preserves the key aspects of our business, but there can be no assurance that we will be successful in our efforts. If we do not effectively integrate Laboratory Services MSO, the effectiveness of our business growth could suffer, and our reputation could be harmed, each of which could adversely impact our business, financial condition and results of operations.
17
The success of our business will depend, in part, on our ability to realize our anticipated benefits and opportunities from the acquisition. We can provide no assurance that the anticipated benefits of the Laboratory Services MSO Acquisition will be fully realized in the time frame anticipated or at all. The failure to meet the challenges involved in integrating the two businesses could cause an interruption of business activities, an increase in operating costs or lower anticipated financial performance. Our failure to achieve the anticipated and the potential benefits underlying our reasons for the Laboratory Services MSO Acquisition could have a material adverse impact on our business, financial condition and results of operations.
We must effectively manage the growth of our operations, or our company will suffer.
To manage our growth, we believe we must continue to implement and improve our services and products. We may not have adequately evaluated the costs and risks associated with our planned expansion, and our systems, procedures, and controls may not be adequate to support our operations. In addition, our management may not be able to achieve the rapid execution necessary to successfully offer our products and services and implement our business plan on a profitable basis. The success of our future operating activities will also depend upon our ability to expand our support system to meet the demands of our growing business. Any failure by our management to effectively anticipate, implement, and manage changes required to sustain our growth would have a material adverse effect on our business, financial condition, and results of operations.
Our revenue and results of operations may suffer if we are unable to attract new clients, continue to engage existing clients, or sell additional products and services.
We presently derive our revenue from providing medical related consulting services to related parties and generating rental revenue from our income-producing real estate property in New Jersey. Our growth therefore depends on our ability to attract new clients, maintain existing clients and properties and sell additional products and services to existing clients. This depends on our ability to understand and anticipate market and pricing trends and our clients’ needs and our ability to deliver consistent, reliable, high-quality services. Our failure to engage new clients, continue to re-engage with our existing clients or cross-sell additional services could materially and adversely affect our operating results.
Our prospects will suffer if we are not able to hire, train, motivate, manage, and retain a significant number of highly skilled employees.
We only recently commenced business and we presently generate medical related consulting services from related parties and generate rental revenue from our income-producing real estate property in New Jersey. On the consulting side, Wenzhao Lu, our Chairman and significant shareholder, is the Chairman of each of the clients in which we have provided consulting services. Our future success depends upon our ability to hire, train, motivate, manage, and retain a significant number of highly skilled employees, particularly research analysts, technical experts, and sales and marketing staff. We will experience competition for professional personnel in each of our business lines. Hiring, training, motivating, managing, and retaining employees with the skills we need is time consuming and expensive. Any failure by us to address our staffing needs in an effective manner could hinder our ability to continue to provide high-quality products and services and to grow our business.
Potential liability claims may adversely affect our business.
Our services, which may include recommendations and advice to organizations regarding complex business and operational processes and regulatory and compliance issues may give rise to liability claims by our clients or by third parties who bring claims against our clients. Healthcare organizations often are the subject of regulatory scrutiny and litigation, and we also may become the subject of such litigation based on our advice and services. Any such litigation, whether or not resulting in a judgment against us, may adversely affect our reputation and could have a material adverse effect on our financial condition and results of operations. We may not have adequate insurance coverage for claims against us.
18
In accordance with our strategic development policy, we may invest in companies for strategic reasons and may not realize a return on our investments.
From time to time, we may make investments in companies. These investments may be for strategic objectives to support our key business initiatives but may also be standalone investments or acquisitions. Such investments or acquisitions could include equity or debt instruments in private companies, many of which may not be marketable at the time of our initial investment. These companies may range from early-stage companies that are often still defining their strategic direction to more mature companies with established revenue streams and business models. The success of these companies may depend on product development, market acceptance, operational efficiency, and other key business factors. The companies in which we invest may fail because they may not be able to secure additional funding, obtain favorable investment terms for future financings, or take advantage of liquidity events such as public offerings, mergers, and private sales. If any of these private companies fails, we could lose all or part of our investment in that company. If we determine that impairment indicators exist and that there are other-than-temporary declines in the fair value of the investments, we may be required to write down the investments to their fair value and recognize the related write-down as an investment loss.
We face intense competition which could cause us to lose market share.
In the healthcare markets in which we operate, we will compete with large healthcare providers who have more significant financial resources, established market positions, long-standing relationships, and who have more significant name recognition, technical, marketing, sales, distribution, financial and other resources than we do. The resources available to our competitors to develop new services and products and introduce them into the marketplace exceed the resources currently available to us. This intense competitive environment may require us to make changes in our services, products, pricing, licensing, distribution, or marketing to develop a market position.
If we fail to comply with our obligations in the agreements under which we license intellectual property rights from third parties or otherwise experience disruptions to our business relationships with our licensors, we could lose intellectual property rights that are important to our business.
We are party to a research agreement with the Massachusetts Institute of Technology (“MIT”) for development of chimeric antigen receptor (CAR) technology. MIT has granted us options to non-exclusively or exclusively license MIT inventions arising under this research agreement. We may need to negotiate commercially reasonable terms and conditions with MIT to advance our research and development activities or allow the commercialization of CAR technology or any other product candidates we may identify and pursue.
Avalon GloboCare and Arbele Limited (“Arbele”) are parties to the joint venture Avactis Biosciences, Inc. (“Avactis”) for development of AVA-011, a mRNA-based dual anti-CD19-CD22 CAR-T cell therapy candidate. Arbele has granted Avactis an exclusive license to its rights in this technology. We and Arbele may need to obtain additional licenses from others to advance our research and development activities or allow the commercialization of mRNA-based CAR technology or any other product candidates we may identify and pursue.
The Company formed a strategic partnership with HydroPeptide, LLC, a leading epigenetics skin care company, to engage in co-development and commercialization of a series of clinical-grade, exosome-based cosmeceutical and orthopedic products. As part of this agreement, the Company signed a three-way Material Transfer Agreement between Avalon GloboCare, HydroPeptide and the University of Pittsburgh Medical Center.
19
The Company and the University of Pittsburgh of the Commonwealth System of Higher Education (the “University”) entered into a Corporate Research Agreement (the “University Agreement”). Pursuant to the University Agreement, for a term of two years the University agreed to use its reasonable efforts to perform academic research funded by the Company in connection with the development of point-of-care modular autonomous processing system to generate clinical-grade AVA-011, a RNA-based chimeric antigen receptor (CAR) T-cell therapy candidate (the “Project”) subject to the appointment of Dr. Yen Michael S. Hsu as Principal Investigator.
Our agreements with MIT, Hydropeptide, University of Pittsburg and Arbele impose, and we expect that future agreements will impose, various development, diligence, commercialization, or other obligations on AVAR and us. In spite of our efforts, these partners may conclude that we have materially breached its obligations under such agreements and might therefore terminate the agreements, thereby removing or limiting our ability or our subsidiary AVAR’s ability to develop and commercialize products and technology covered by these license agreements. If these in-licenses are terminated, or if the underlying patents fail to provide the intended exclusivity, competitors or other third parties would have the freedom to seek regulatory approval of, and to market, products identical to ours and we may be required to cease our development and commercialization of CAR or exosome technology or other product candidates that we may identify. Any of the foregoing could have a material adverse effect on our competitive position, business, financial conditions, results of operations, and prospects.
Moreover, disputes may arise regarding intellectual property subject to a licensing agreement, including:
| ● | the scope of rights granted under the license agreement and other interpretation-related issues; |
| ● | the extent to which our product candidates, technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement; |
| ● | the sublicensing of patent and other rights under our collaborative development relationships; |
| ● | our diligence obligations under the license agreement and what activities satisfy those diligence obligations; |
| ● | the inventorship and ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and |
| ● | the priority of invention of patented technology. |
In addition, the agreements under which we currently license intellectual property or technology from third parties are complex, and certain provisions in such agreements may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could narrow what we believe to be the scope of our rights to the relevant intellectual property or technology, or increase what we believe to be our financial or other obligations under the relevant agreement, either of which could have a material adverse effect on our business, financial condition, results of operations, and prospects. Moreover, if disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on commercially acceptable terms, we may be unable to successfully develop and commercialize the affected product candidates, which could have a material adverse effect on our business, financial conditions, results of operations, and prospects.
20
We may face uncertainty and difficulty in obtaining and enforcing our patents and other proprietary rights.
There can be no assurance that any patent applications we file or license will be approved, or that challenges will not be instituted against the validity or enforceability of any patent licensed-in or owned by us. Our pending and future patent applications may not result in patents being issued that protect our product candidates, in whole or in part, or which effectively prevent others from commercializing competitive product candidates. Even if our patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our patents by developing similar or alternative product candidates in a non-infringing manner. The cost of litigation to uphold the validity and prevent infringement of a patent is substantial. Furthermore, there can be no assurance that others will not independently develop substantially equivalent technologies not covered by patents to which we have rights or obtain access to our know-how. In addition, the laws of certain countries may not adequately protect our intellectual property. Our competitors may possess or obtain patents on products or processes that are necessary or useful to the development, use, or manufacture of our product candidates. There can also be no assurance that our proposed technology will not infringe upon patents or proprietary rights owned by others, with the result that others may bring infringement claims against us and require us to license such proprietary rights, which may not be available on commercially reasonable terms, if at all. Any such litigation, if instituted, could have a material adverse effect, potentially including monetary penalties, diversion of management resources, and injunction against continued manufacture, use, or sale of certain products or processes.
We rely upon non-patented proprietary know-how. There can be no assurance that we can adequately protect our rights in such non-patented proprietary know-how, or that others will not independently develop substantially equivalent proprietary information or techniques or gain access to our proprietary know-how. Any of the foregoing events could have a material adverse effect on us. In addition, if any of our trade secrets, know-how or other proprietary information were to be disclosed, or misappropriated, the value of our trade secrets, know-how and other proprietary rights would be significantly impaired and our business and competitive position would suffer.
In September 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law. These include provisions that affect the way patent applications will be prosecuted and may also affect patent litigation. In particular, under the Leahy-Smith Act, the United States transitioned in March 2013 to a “first to file” system in which the first inventor to file a patent application will be entitled to the patent. Third parties are allowed to submit prior art before the issuance of a patent by the U.S. Patent and Trademark Office, or USPTO, and may become involved in opposition, derivation, post-grant and inter partes review, or interference proceedings challenging our patent rights. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights, which could adversely affect our competitive position.
The USPTO has developed new and untested regulations and procedures to govern the full implementation of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the “first-to-file” provisions, only became effective in March 2013. The Leahy-Smith Act has also introduced procedures that may make it easier for third parties to challenge issued patents, as well as to intervene in the prosecution of patent applications. Finally, the Leahy-Smith Act contains new statutory provisions that still require the USPTO to issue new regulations for their implementation, and it may take the courts years to interpret the provisions of the new statute. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. The Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on our product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States may be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we do not obtain patent protection to develop their own products and may also export infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our products and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
21
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets, and other intellectual property protection, particularly those relating to biotechnology products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions, whether or not successful, could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Patent terms may be inadequate to protect our competitive position on our product candidates for an adequate amount of time.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our product candidates are obtained, once the patent life has expired, we may be open to competition from competitive products, including generics or biosimilars. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, any patents we may obtain may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
Obtaining and maintaining patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and any patent protection we may obtain in the future could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and/or applications will be due to be paid to the USPTO and various governmental patent agencies outside of the United States in several stages over the lifetime of the patents and/or applications. The USPTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. There are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, our competitors might be able to enter the market and this circumstance would have a material adverse effect on our business.
It is difficult and costly to protect our proprietary rights, and we may not be able to ensure their protection. If we fail to protect or enforce our intellectual property rights adequately or secure rights to patents of others, the value of our intellectual property rights would diminish.
Our commercial viability will depend in part on obtaining and maintaining patent protection and trade secret protection of our product candidates, and the methods used to manufacture them, as well as successfully defending these patents against third-party challenges. Our ability to stop third parties from making, using, selling, offering to sell, or importing our products is dependent upon the extent to which we obtain rights under valid and enforceable patents or trade secrets that cover these activities.
The patent positions of pharmaceutical and biopharmaceutical companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in biopharmaceutical patents has emerged to date in the United States. The biopharmaceutical patent situation outside the United States is even more uncertain. Changes in either the patent laws or in interpretations of patent laws in the United States and other countries may diminish the value of our intellectual property. Accordingly, we cannot predict the breadth of claims that may be allowed or enforced in the patents we own. Further, if any of our patents are deemed invalid and unenforceable, it could impact our ability to commercialize or license our technology.
22
The degree of future protection for our proprietary rights is uncertain because legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep our competitive advantage. For example:
| ● | others may be able to make products that are similar to our product candidates but that are not covered by the claims of any patents; |
| ● | we might not have been the first to make the inventions covered by any issued patents or patent applications; |
| ● | we might not have been the first to file patent applications for these inventions; |
| ● | it is possible that any patent applications we own or license will not result in issued patents; |
| ● | any issued patents may not provide us with any competitive advantages, or may be held invalid or unenforceable as a result of legal challenges by third parties; |
| ● | we may not develop additional proprietary technologies that are patentable or protectable under trade secrets law; or |
| ● | the patents of others may have an adverse effect on our business. |
We also may rely on trade secrets to protect our technology, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. Although we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors, outside scientific collaborators, and other advisors may unintentionally or willfully disclose our information to competitors. In addition, courts outside the United States are sometimes less willing to protect trade secrets. Moreover, our competitors may independently develop equivalent knowledge, methods, and know-how.
We may be subject to claims challenging the inventorship of patents and other intellectual property.
We or our licensors may be subject to claims that former employees, collaborators or other third parties have an interest as an inventor or co-inventor in intellectual property we own or license. For example, we or our licensors may have inventorship disputes arise from conflicting obligations of employees, consultants or others who are involved in developing our product candidates. We may be subject to claims by third parties asserting that our licensors, employees or we have misappropriated their intellectual property, or claiming ownership of what we regard as our own intellectual property. Litigation may be necessary to defend against these and other claims challenging inventorship or our or our licensors’ ownership of our owned or in-licensed patents, trade secrets or other intellectual property. If we or our licensors fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, intellectual property that is important to our product candidates. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
If any of our trade secrets, know-how or other proprietary information is disclosed, the value of our trade secrets, know-how and other proprietary rights would be significantly impaired and our business and competitive position would suffer.
Our viability also depends upon the skills, knowledge and experience of our scientific and technical personnel, and our consultants and advisors. To help protect our proprietary know-how and our inventions for which patents may be unobtainable or difficult to obtain, we rely on trade secret protection and confidentiality agreements. To this end, we require all of our employees, consultants, advisors and contractors to enter into agreements which prohibit unauthorized disclosure and use of confidential information and, where applicable, require disclosure and assignment to us of the ideas, developments, discoveries and inventions important to our business. These agreements are often limited in duration and may not provide adequate protection for our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure or the lawful development by others of such information. There is no assurance that such agreements will be honored by such parties or enforced in whole or part by the courts. We cannot be certain that others will not gain access to these trade secrets or that our patents will provide adequate protection. Others may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets. In addition, enforcing a claim that a third party illegally obtained and is using any of our trade secrets is expensive and time consuming, and the outcome is unpredictable. If any of our trade secrets, know-how or other proprietary information is improperly disclosed, the value of our trade secrets, know-how and other proprietary rights would be significantly impaired and our business and competitive position would suffer.
23
We may incur substantial costs as a result of litigation or other proceedings relating to patent and other intellectual property rights and we may be unable to protect our rights to, or use of, our technology.
If we choose to go to court to stop a third party from using the inventions claimed in our patents, that individual or company has the right to ask the court to rule that such patents are invalid and/or should not be enforced against that third party. These lawsuits are expensive and would consume time and other resources, even if we were successful in discontinuing the infringement of our patents. In addition, there is a risk that the court will decide that these patents are not valid and that we do not have the right to stop the other party from using the inventions. There is also the risk that, even if the validity of these patents is upheld, the court will refuse to stop the other party on the ground that such other party’s activities do not infringe our rights to these patents. In addition, the U.S. Supreme Court has in the past invalidated tests used by the USPTO in granting patents over the past 20 years. As a consequence, issued patents may be found to contain invalid claims according to the newly revised standards. Some of our own patents may be subject to challenge and subsequent invalidation in a variety of post-grant proceedings, particularly inter partes review, before the USPTO or during litigation under the revised criteria, which make it more difficult to defend the validity of claims in already issued patents.
Furthermore, a third party may claim that we or our manufacturing or commercialization partners are using inventions covered by the third party’s patent rights and may go to court to stop us from engaging in our normal operations and activities, including making or selling our product candidates. These lawsuits are costly and could affect our results of operations and divert the attention of managerial and technical personnel. There is a risk that a court could decide that we or our commercialization partners are infringing the third party’s patents and order us or our partners to stop the activities covered by the patents. In addition, there is a risk that a court could order us or our partners to pay the other party damages for having violated the other party’s patents. The biotechnology industry has produced a proliferation of patents, and it is not always clear to industry participants, including us, which patents cover various types of products, manufacturing processes or methods of use. The coverage of patents is subject to interpretation by the courts, and the interpretation is not always uniform. If we are sued for patent infringement, we would need to demonstrate that our products, manufacturing processes or methods of use either do not infringe the patent claims of the relevant patent and/or that the patent claims are invalid, and we may not be able to do this. Proving invalidity, in particular, is difficult since it requires a showing of clear and convincing evidence to overcome the presumption of validity enjoyed by issued patents.
As some patent applications in the United States may be maintained in secrecy until the patents are issued, because patent applications in the United States and many foreign jurisdictions are typically not published until eighteen months after filing, and because publications in the scientific literature often lag behind actual discoveries, we cannot be certain that others have not filed patent applications for technology covered by our issued patents or our pending applications, or that we were the first to invent the technology. Our competitors may have filed, and may in the future file, patent applications covering technology similar to ours. Any such patent applications may have priority over our patent applications or patents, which could further require us to obtain rights to issued patents covering such technologies. If another party has filed a United States patent application on inventions similar to ours, we may have to participate in an interference proceeding declared by the USPTO to determine priority of invention in the United States. The costs of these proceedings could be substantial, and it is possible that such efforts would be unsuccessful if, unbeknownst to us, the other party had independently arrived at the same or similar invention prior to our own invention, resulting in a loss of our U.S. patent position with respect to such inventions.
Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. In addition, any uncertainties resulting from the initiation and continuation of any litigation or inter partes review proceedings could have a material adverse effect on our ability to raise the funds necessary to continue our operations.
24
Some jurisdictions in which we operate have enacted legislation which allows members of the public to access information under statutes similar to the U.S. Freedom of Information Act. Even though we believe our information would be excluded from the scope of such statutes, there are no assurances that we can protect our confidential information from being disclosed under the provisions of such laws. If any confidential or proprietary information is released to the public, such disclosures may negatively impact our ability to protect our intellectual property rights.
Breaches or compromises of our information security systems or our information technology systems or infrastructure could result in exposure of private information, disruption of our business and damage to our reputation, which could harm our business, results of operation and financial condition.
We utilize information security and information technology systems and websites that allow for the secure storage and transmission of proprietary or private information regarding our clients, patients, employees, vendors and others, including individually identifiable health information. A security breach of our network, hosted service providers, or vendor systems, may expose us to a risk of loss or misuse of this information, litigation and potential liability. Hackers and data thieves are increasingly sophisticated and operate large-scale and complex automated attacks, including on companies within the healthcare industry. Although we believe that we take appropriate measures to safeguard sensitive information within our possession, we may not have the resources or technical sophistication to anticipate or prevent rapidly-evolving types of cyber-attacks targeted at us, our clients, our patients, or others who have entrusted us with information. Actual or anticipated attacks may cause us to incur costs, including costs to deploy additional personnel and protection technologies, train employees, and engage third-party experts and consultants. We invest in industry standard security technology to protect personal information. Advances in computer capabilities, new technological discoveries, or other developments may result in the technology used by us to protect personal information or other data being breached or compromised. To our knowledge, we have not experienced any material breach of our cybersecurity systems. If our or our third-party service provider systems fail to operate effectively or are damaged, destroyed, or shut down, or there are problems with transitioning to upgraded or replacement systems, or there are security breaches in these systems, any of the aforementioned could occur as a result of natural disasters, software or equipment failures, telecommunications failures, loss or theft of equipment, acts of terrorism, circumvention of security systems, or other cyber-attacks, we could experience delays or decreases in revenue, and reduced efficiency of our operations. Additionally, any of these events could lead to violations of privacy laws, loss of customers, or loss, misappropriation or corruption of confidential information, trade secrets or data, which could expose us to potential litigation, regulatory actions, sanctions or other statutory penalties, any or all of which could adversely affect our business, and cause us to incur significant losses and remediation costs.
We may be exposed to liabilities under the Foreign Corrupt Practices Act, and any determination that we violated the Foreign Corrupt Practices Act or Chinese anti-corruption law could have a material adverse effect on our business.
We are subject to the Foreign Corrupt Practice Act, or FCPA, and other laws that prohibit improper payments or offers of payments to foreign governments and their officials and political parties by U.S. persons and issuers as defined by the statute, for the purpose of obtaining or retaining business. We have operations and agreements with third parties where corruption may occur. It is our policy to implement safeguards to prevent these practices by our employees. However, our existing safeguards and any future improvements may prove to be less than effective, and the employees, consultants, sales agents or distributors of our company may engage in conduct for which we might be held responsible.
Violations of the FCPA or other anti-corruption laws may result in severe criminal or civil sanctions, and we may be subject to other liabilities, which could negatively affect our business, operating results and financial condition. In addition, the United States government may seek to hold our company liable for successor liability FCPA violations committed by companies in which we invest or that we acquire.
25
Risk Factors Related to our Laboratory Services MSO Business
Continued changes in healthcare reimbursement models and products (e.g., health insurance exchanges), changes in government payment and reimbursement systems, or changes in payer mix, including an increase in third-party benefits management and value-based payment models, could have a material adverse effect on our revenues, profitability and cash flow.
Diagnostic testing services are billed to managed care organizations (MCOs), Medicare, Medicaid, physicians and physician groups, hospitals, patients and employer groups. Most testing services are billed to a party other than the physician or other authorized person who ordered the test. Increases in the percentage of services billed to government and MCOs could have an adverse effect on our revenues. Although we currently do not provide any “in network” laboratory services, our plan is to begin providing such services in the near future.
These organizations have different contracting philosophies, which are influenced by the design of their products. Some MCOs contract with a limited number of clinical laboratories and engage in direct negotiation of rates. Other MCOs adopt broader networks with generally uniform fee structures for participating clinical laboratories. In some cases, those fee structures are specific to independent clinical laboratories, while the fees paid to hospital-based and physician-office laboratories may be different, and are typically higher. MCOs may also offer Managed Medicare or Managed Medicaid plans. In addition, an increasing number of MCOs are implementing, directly or through third parties, various types of laboratory benefit management programs that may include laboratory networks, utilization management tools (such as prior authorization and/or prior notification), and claims edits, which may impact coverage or reimbursement for commercial laboratory tests. Some of these programs address commercial laboratory testing broadly, while others are focused on certain types of testing such as molecular, genetic and toxicology testing. An increase in the use of such programs could lead to increased denial of claims, extended appeals, and reduced revenue.
Our ability to attract and retain MCOs is critical given the impact of healthcare reform, related products and expanded coverage (e.g. health insurance exchanges and Medicaid expansion) and evolving value-based care and risk-based reimbursement delivery models (e.g., accountable care organizations (ACOs) and Independent Physician Associations (IPAs)).
A portion of the managed care fee-for-service revenues is collectible from patients in the form of deductibles, coinsurance and copayments. As patient cost-sharing has been increasing, our collections may be adversely impacted.
In addition, Medicare and Medicaid and private insurers have increased their efforts to control the cost, utilization and delivery of healthcare services, including commercial laboratory services. Measures to regulate healthcare delivery in general, and clinical laboratories in particular, have resulted in reduced prices, added costs and decreased test utilization for the commercial laboratory industry by increasing complexity and adding new regulatory and administrative requirements. Pursuant to legislation passed in late 2003, the percentage of Medicare beneficiaries enrolled in Managed Medicare plans has increased. The percentage of Medicaid beneficiaries enrolled in Managed Medicaid plans has also increased; however, changes to, or repeal of, the Patient Protection and Affordable Care Act (ACA) may continue to affect coverage, reimbursement, and utilization of laboratory services, as well as administrative requirements, in ways that are currently unpredictable. Further healthcare reform could adversely affect laboratory reimbursement from Medicare, Medicaid or commercial carriers.
We expect the efforts to impose reduced reimbursement, more stringent payment policies, and utilization and cost controls by government and other payers to continue. If our laboratory services business cannot offset additional reductions in the payments it receives for its services by reducing costs, increasing test volume, and/or introducing new services and procedures, it could have a material adverse effect on our revenues, profitability and cash flows. In 2014, Congress passed the Protecting Access to Medicare Act (PAMA), requiring Medicare to change the way payment rates are calculated for tests paid under the Clinical Laboratory Fee Schedule (CLFS), and to base the payment on the weighted median of rates paid by private payers. On June 23, 2016, CMS issued a final rule to implement PAMA that required applicable laboratories, including our laboratory services business, to begin reporting their test-specific private payer payment amounts to CMS during the first quarter of 2017. CMS exercised enforcement discretion to permit reporting for an additional 60 days, through May 30, 2017. CMS used that private market data to calculate weighted median prices for each test (based on applicable current procedural technology (CPT) codes) to represent the new CLFS rates beginning in 2018, subject to certain phase-in limits. For 2018-2020, a test price could not be reduced by more than 10% per year. As a result of provisions included within the CARES Act, PAMA rate reductions for 2021 were suspended. As a result of the Protecting Medicare and American Farmers from Sequester Cuts Act that became law in December 2021, the data reporting requirements and Medicare reimbursement cuts that would have occurred under PAMA in 2022 were delayed by one additional year. As a result of the Consolidated Appropriations Act, 2023, which became law in December 2022, the data reporting requirements and Medicare reimbursement cuts that would have occurred under PAMA in 2023 were delayed by one additional year.
26
For 2024-2026, a test price cannot be reduced by more than 15.0% per year. The process of data reporting and repricing will be repeated every three years for Clinical Diagnostic Laboratory Tests (CDLTs) beginning in 2024. CFLS rates for 2027 and subsequent periods will not be subject to phase-in limits. The phase-in of rates for CDLTs established in 2018 will resume in 2024. New CLFS rates will be established in 2025 based on data from 2019 to be reported in 2024. New CLFS rates will be established in 2028 based on data from 2026 to be reported in 2027 CLFS rates for Advanced Diagnostic Laboratory Tests (ADLTs) will be updated annually.
CMS published its initial proposed CLFS rates under PAMA for 2018-2020 on September 22, 2017. Following a public comment period, CMS made adjustments and published final CLFS rates for 2018-2020 on November 17, 2017, with additional adjustments published on December 1, 2017. 2021, 2022 and 2023 PAMA rates were frozen as described above.
Healthcare reform legislation also contains numerous regulations that will require us, as an employer, to implement significant process and record-keeping changes to be in compliance. These changes increase the cost of providing healthcare coverage to employees and their families. Given the limited release of regulations to guide compliance, as well as potential changes to the ACA, the exact impact to employers, including us, is uncertain.
Government payers, such as Medicare and Medicaid, have taken steps to reduce the utilization and reimbursement of healthcare services, including clinical testing services.
Although we currently do not provide any laboratory services that are billed through Medicare or Medicaid, we plan to do so in the near future. At that time, we will face efforts by government payers to reduce utilization of and reimbursement for diagnostic information services. One example of this is increased use of prior authorization requirements. We expect efforts to reduce reimbursements, to impose more stringent cost controls and to reduce utilization of clinical test services will continue.
Pursuant to PAMA, reimbursement rates for many clinical laboratory tests provided under Medicare were reduced from 2018 - 2020. PAMA calls for further revision of the Medicare CLFS for years after 2020, based on future surveys of market rates; reimbursement rate reduction from 2024-26 is capped by PAMA at 15% annually. PAMA’s next data collection and reporting period have been delayed, most recently by federal legislation adopted in December 2022, which further delayed the reimbursement rate reductions and reporting requirements until January 1, 2024.
In addition, CMS has adopted policies limiting or excluding coverage for clinical tests that we perform. We also expect in the future to provide physician services that are reimbursed by Medicare under a physician fee schedule, which is subject to adjustment on an annual basis. Medicaid reimbursement varies by state and is subject to administrative and billing requirements and budget pressures.
In addition, over the last several years, the federal government has expanded its contracts with private health insurance plans for Medicare beneficiaries, called “Medicare Advantage” programs, and has encouraged such beneficiaries to switch from the traditional programs to the private programs. There has been growth of health insurance plans offering Medicare Advantage programs, and of beneficiary enrollment in these programs. States have mandated that Medicaid beneficiaries enroll in private managed care arrangements. In addition, state budget pressures have encouraged states to consider several courses of action that may impact our business, such as delaying payments, reducing reimbursement, restricting coverage eligibility, denying claims and service coverage restrictions. Further, CMS has set goals for value-based reimbursement to be achieved by 2030.
Reimbursement for Medicare services also is subject to annual reduction under the Budget Control Act of 2011, and the Statutory Pay-As-You-Go Act of 2010.
From time to time, the federal government has considered whether competitive bidding could be used to provide clinical testing services for Medicare beneficiaries while maintaining quality and access to care. Congress periodically considers cost-saving initiatives. These initiatives have included coinsurance for clinical testing services, co-payments for clinical testing and further laboratory physician fee schedule reductions.
Other steps taken to reduce utilization and reimbursement include requirements to obtain diagnosis codes to obtain payment, increased documentation requirements, limiting the allowable number of tests or ordering frequency, expanded prior authorization programs and otherwise increasing payment denials.
Steps to reduce utilization and reimbursement also discourage innovation and access to innovative solutions that we may offer.
27
Health plans and other third parties have taken steps to reduce the utilization and reimbursement of health services, including clinical testing services.
We face efforts by non-governmental third-party payers, including health plans, to reduce utilization of and reimbursement for clinical testing services. Examples include increased use of prior authorization requirements and increased denial of coverage for services. There is increased market activity regarding alternative payment models, including bundled payment models. We expect continuing efforts by third-party payers, including in their rules, practices and policies, to reduce reimbursements, to impose more stringent cost controls and to reduce utilization of clinical testing services. ACOs and Independent Delivery Networks (IDNs), including hospitals and hospital health systems, also may undertake efforts to reduce utilization of, or reimbursement for, diagnostic information services.
The healthcare industry has experienced a trend of consolidation among health insurance plans, resulting in fewer but larger insurance plans with significant bargaining power to negotiate fee arrangements with clinical testing providers. The increased consolidation among health plans also has increased pricing transparency, insurer bargaining power and the potential adverse impact of ceasing to be a contracted provider with an insurer. Health plans, and independent physician associations, may demand that clinical testing providers accept discounted fee structures or assume all or a portion of the financial risk associated with providing testing services to their members through capitated payment arrangements. Some health plans also are reviewing test coding, evaluating coverage decisions and requiring preauthorization of certain testing. There are also an increasing number of patients enrolling in consumer driven products and high deductible plans that involve greater patient cost-sharing.
Other steps taken to reduce utilization and reimbursement include requirements to obtain diagnosis codes to obtain payment, increased documentation requirements, limiting the allowable number of tests or ordering frequency, expanded prior authorization programs and otherwise increasing payment denials.
Steps to reduce utilization and reimbursement also discourage innovation and access to innovative solutions that we may offer.
The clinical testing business is highly competitive, and if we fail to provide an appropriately priced level of service or otherwise fail to compete effectively it could have a material adverse effect on our revenues and profitability.
The laboratory testing industry is fragmented and highly competitive. We primarily compete with three types of clinical testing providers: commercial clinical laboratories IDN-affiliated laboratories and physician-office laboratories. Our largest commercial clinical laboratory competitors are Quest Diagnostic Laboratories and Laboratory Corporation of America. In addition, we compete with many smaller regional and local commercial clinical laboratories, specialized advanced laboratories and providers of consumer-initiated testing. There also has been a trend among physician practices to establish their own histology laboratory capabilities and/or bring pathologists into their practices, thereby reducing referrals from these practices and increasing the competitive position of these practices.
The commercial laboratory business is intensely competitive both in terms of price and service. Pricing of laboratory testing services is often one of the most significant factors used by physicians, third-party payers and consumers in selecting a laboratory. As a result of significant consolidation in the commercial laboratory industry, larger commercial laboratory providers are able to increase cost efficiencies afforded by large-scale automated testing. This consolidation results in greater price competition. Our laboratory services business may be unable to increase cost efficiencies sufficiently, if at all, and as a result, its net earnings and cash flows could be negatively impacted by such price competition. We may face increased competition from health system laboratories, due to physicians within those systems directing their testing to the health system laboratory and away from us, and as those laboratories seek to expand their testing volume from unaffiliated physicians in their service areas. We may also face competition from companies that do not comply with existing laws or regulations or otherwise disregard compliance standards in the industry. Additionally, we may also face changes in fee schedules, competitive bidding for laboratory services, or other actions or pressures reducing payment schedules as a result of increased or additional competition. These competitive pressures may affect the attractiveness or profitability of our laboratory services business, and could adversely affect our financial results.
28
The diagnostic information services industry also is faced with changing technology and new product introductions. Competitors may compete using advanced technology, including technology that enables more convenient or cost-effective testing. Digital pathology, still in an emerging state, is an example of this. Competitors also may compete on the basis of new service offerings. Competitors also may offer testing to be performed outside of a commercial clinical laboratory, such as (1) point-of-care testing that can be performed by physicians in their offices; (2) advanced testing that can be performed by IDNs in their own laboratories; and (3) home testing that can be carried out without requiring the services of outside providers.
Failure to obtain and retain new customers, the loss of existing customers or material contracts, or a reduction in services or tests ordered or specimens submitted by existing customers, or the inability to retain existing and/or create new relationships with health systems could impact our ability to successfully grow our business.
To maintain and grow its business, we need to obtain and retain new customers and business partners. In addition, a reduction in tests ordered or specimens submitted by existing customers, a decrease in demand for our services from existing customers, or the loss of existing contracts, without offsetting growth in its customer base, could impact our ability to successfully grow its business and could have a material adverse effect on our revenues and profitability. We compete primarily on the basis of the quality of services, reporting and information systems, reputation in the medical community, the pricing of services and ability to employ qualified personnel. Our failure to successfully compete on any of these factors could result in the loss of existing customers, an inability to gain new customers and a reduction in our business.
Discontinuation or recalls of existing testing products; failure to develop or acquire licenses for new or improved testing technologies; or our customers using new technologies to perform their own tests could adversely affect our business.
From time to time, manufacturers discontinue or recall reagents, test kits or instruments used by us to perform laboratory testing. Such discontinuations or recalls could adversely affect our costs, testing volume and revenue.
The commercial laboratory industry is subject to changing technology and new product introductions. If we are unable to license new or improved technologies to expand its esoteric testing operations, its testing methods may become outdated when compared with our competition, and testing volume and revenue may be materially and adversely affected.
In addition, advances in technology may lead to the development of more cost-effective technologies such as point-of-care testing equipment that can be operated by physicians or other healthcare providers (including physician assistants, nurse practitioners and certified nurse midwives, generally referred to herein as physicians) in their offices or by patients themselves without requiring the services of freestanding clinical laboratories. Development of such technology and its use by our customers could reduce the demand for its laboratory testing services and the utilization of certain tests offered by us and negatively impact its revenues.
Currently, most commercial laboratory testing is categorized as high or moderate complexity, and thereby is subject to extensive and costly regulation under Clinical Laboratory Improvement Act (CLIA). The cost of compliance with CLIA makes it impractical for most physicians to operate clinical laboratories in their offices, and other laws limit the ability of physicians to have ownership in a laboratory and to refer tests to such a laboratory. Manufacturers of laboratory equipment and test kits could seek to increase their sales by marketing point-of-care laboratory equipment to physicians and by selling test kits approved for home or physician office use to both physicians and patients. Diagnostic tests approved for home use are automatically deemed to be “waived” tests under CLIA and may be performed in physician office laboratories as well as by patients in their homes with minimal regulatory oversight. Other tests meeting certain FDA criteria also may be classified as “waived” for CLIA purposes. The FDA has regulatory responsibility over instruments, test kits, reagents and other devices used by clinical laboratories, and it has taken responsibility from the U.S. Centers for Disease Control and Prevention for classifying the complexity of tests for CLIA purposes. Increased approval of “waived” test kits could lead to increased testing by physicians in their offices or by patients at home, which could affect our market for laboratory testing services and negatively impact its revenues.
29
Changes or disruption in services supplies, or transportation provided by third parties have impacted and could continue to impact or adversely affect our business.
We depend on third parties to provide supplies and services critical to our laboratory testing business. We are heavily reliant on third-party ground and air travel for transport of clinical trial and diagnostic testing supplies and specimens, research products, and people. A significant disruption to these travel systems, or our access to them, could have a material adverse effect on our business. We are also reliant on an extensive network of third-party suppliers and vendors of certain services and products, including for certain animal populations. Disruptions to the continued supply, or increases in costs, of these services, products, or animal populations may arise from export/import restrictions or embargoes, political or economic instability, pressure from animal rights activists, adverse weather, natural disasters, public health crises, transportation disruptions, cyber-attacks, or other causes, as well as from termination of relationships with suppliers or vendors for their failure to follow our performance standards and requirements. Disruption of supply and services has impacted and could continue to impact or have a material adverse effect on our business.
Continued and increased consolidation of pharmaceutical, biotechnology and medical device companies, health systems, physicians and other customers could adversely affect our business.
Many healthcare companies and providers, including pharmaceutical, biotechnology and medical device companies, health systems and physician practices are consolidating through mergers, acquisitions, joint ventures and other types of transactions and collaborations. In addition to these more traditional horizontal mergers that involve entities that previously competed against each other, the healthcare industry is experiencing an increase in vertical mergers, which involve entities that previously did not offer competing goods or services. As the healthcare industry consolidates, competition to provide goods and services may become more intense, and vertical mergers may give those combined companies greater control over more aspects of healthcare, including increased bargaining power. This competition and increased customer bargaining power may adversely affect the price and volume of our services.
In addition, as the broader healthcare industry trend of consolidation continues, including the acquisition of physician practices by health systems, relationships with hospital-based health systems and integrated delivery networks are becoming more important. Our laboratory services business’ inability to retain its existing relationships with physicians if they become part of healthcare systems and networks and/or to create new relationships could impact its ability to successfully grow.
Changes, including changes in interpretation, in payer regulations, policies or approvals, or changes in laws, regulations or policies in the U.S. or globally, may adversely affect us.
U.S. and state government payers, such as Medicare and Medicaid, as well as insurers, including MCOs, have increased their efforts to control the cost, utilization and delivery of healthcare services. From time to time, Congress has considered and implemented changes in Medicare fee schedules in conjunction with budgetary legislation. The first phase of reductions pursuant to PAMA came into effect on January 1, 2018, and will continue annually subject to certain delays in implementation and phase-in limits through 2026, and without limitations for subsequent periods. Further reductions due to changes in policy regarding coverage of tests or other requirements for payment, such as prior authorization, diagnosis code and other claims edits, may be implemented from time to time. Reimbursement for pathology services performed by us is also subject to statutory and regulatory reduction. Reductions in the reimbursement rates and changes in payment policies of other third-party payers may occur as well. Such changes in the past have resulted in reduced payments as well as added costs and have decreased test utilization for the commercial laboratory industry by adding more complex new regulatory and administrative requirements. Further changes in third-party payer regulations, policies, or laboratory benefit or utilization management programs may have a material adverse effect on our business. Actions by federal and state agencies regulating insurance, including healthcare exchanges, or changes in other laws, regulations, or policies may also have a material adverse effect upon our business.
30
Our business could be harmed from the loss or suspension of a license or imposition of a fine or penalties under, or future changes in, or interpretations of, the law or regulations of CLIA, Medicare, Medicaid or other national, state or local agencies in the U.S. and other countries where we operate laboratories currently and in the future.
The commercial laboratory testing industry is subject to extensive U.S. regulation, and many of these statutes and regulations have not been interpreted by the courts. CLIA extends federal oversight to virtually all clinical laboratories operating in the U.S. by requiring that they be certified by the federal government or by a federally approved accreditation agency. The sanction for failure to comply with CLIA requirements may be suspension, revocation or limitation of a laboratory’s CLIA certificate, which is necessary to conduct business, as well as significant fines and/or criminal penalties. In addition, we are subject to regulation under state law. State laws may require that laboratories and/or laboratory personnel meet certain qualifications, specify certain quality controls or require maintenance of certain records. In the future, we may also operate laboratories outside of the U.S. and become subject to laws governing its laboratory operations in the other countries where it operates.
Applicable statutes and regulations could be interpreted or applied by a prosecutorial, regulatory or judicial authority in a manner that would adversely affect our business. Potential sanctions for violation of these statutes and regulations include significant fines and the suspension or loss of various licenses, certificates and authorizations, which could have a material adverse effect on our business. In addition, compliance with future legislation could impose additional requirements on us, which may be costly.
Failure of us or our third-party service providers to comply with privacy and security laws and regulations could result in fines, penalties and damage to our reputation with customers and have a material adverse effect upon our business.
If we and our third-party service providers do not comply with existing or new laws and regulations related to protecting the privacy and security of personal or health information, we could be subject to monetary fines, civil penalties or criminal sanctions.
In the U.S., HIPAA privacy and security regulations, including the expanded requirements under HITECH, establish comprehensive standards with respect to the use and disclosure of protected health information (PHI), by covered entities, in addition to setting standards to protect the confidentiality, integrity and security of PHI.
HIPAA restricts our ability to use or disclose PHI, without patient authorization, for purposes other than payment, treatment or healthcare operations (as defined by HIPAA), except for disclosures for various public policy purposes and other permitted purposes outlined in the privacy regulations. HIPAA and HITECH provide for significant fines and other penalties for wrongful use or disclosure of PHI in violation of the privacy and security regulations, including potential civil and criminal fines and penalties. The regulations establish a complex regulatory framework on a variety of subjects, including:
| ● | the circumstances under which the use and disclosure of PHI are permitted or required without a specific authorization by the patient, including, but not limited to, treatment purposes, activities to obtain payments for our services, and its healthcare operations activities; | |
| ● | a patient’s rights to access, amend and receive an accounting of certain disclosures of PHI; | |
| ● | the content of notices of privacy practices for PHI; | |
| ● | administrative, technical and physical safeguards required of entities that use or receive PHI; and | |
| ● | the protection of computing systems maintaining electronic PHI. |
We have implemented policies and procedures designed to comply with the HIPAA privacy and security requirements as applicable. The privacy and security regulations establish a “floor” and do not supersede state laws that are more stringent. Therefore, we are required to comply with both additional federal privacy and security regulations and varying state privacy and security laws. In addition, federal and state laws that protect the privacy and security of patient information may be subject to enforcement and interpretations by various governmental authorities and courts, resulting in complex compliance issues. For example, we could incur damages under state laws, including pursuant to an action brought by a private party for the wrongful use or disclosure of health information or other personal information.
31
Failure to comply with U.S., state or local environmental, health and safety laws and regulations could result in fines, penalties and loss of licensure, and have a material adverse effect upon us.
We are subject to licensing and regulation under laws and regulations relating to the protection of the environment and human health and safety, including laws and regulations relating to the handling, transportation and disposal of medical specimens, infectious and hazardous waste and radioactive materials, as well as regulations relating to the safety and health of laboratory employees. Failure to comply with these laws and regulations could subject us to denial of the right to conduct business, fines, criminal penalties and/or other enforcement actions that would have a material adverse effect on its business. In addition, compliance with future legislation could impose additional requirements on us that may be costly.
The U.S. healthcare system is evolving and medical laboratory testing market fundamentals are changing, and our business could be adversely impacted if we fail to adapt.
The U.S. healthcare system continues to evolve. Significant change is taking place in the healthcare system. For example, value-based reimbursement is increasing; CMS has set goals for value-based reimbursement to be achieved by 2030. Patients are encouraged to take increased interest in and responsibility for, and often are bearing increased responsibility for payment for, their healthcare. Healthcare industry participants are evolving and consolidating. Healthcare services increasingly are being provided by non-traditional providers (e.g., physician assistants), in non-traditional venues (e.g., retail medical clinics, urgent care centers) and using new technologies (e.g., telemedicine, digital pathology). Utilization of the healthcare system is being influenced by several factors and may result in a decline in the demand for diagnostic information services.
In addition, we believe that clinical testing market fundamentals are changing. We believe that PAMA-driven reimbursement pressure remains a catalyst for structural change in the market. We also believe that health plans and consumers increasingly are focusing on driving better value in laboratory testing services. We expect that the evolution of the healthcare industry will continue, and that industry change is likely to be extensive.
Failure to establish, and perform to, appropriate quality standards, or to assure that the appropriate standard of quality is observed in the performance of our diagnostic information services, could adversely affect the results of our operations and adversely impact our reputation.
The provision of diagnostic information services involves certain inherent risks. The services that we provide are intended to provide information in providing patient care. Therefore, users of our services may have a greater sensitivity to errors than the users of services or products that are intended for other purposes.
Negligence in performing our services can lead to injury or other adverse events. We may be sued under physician liability or other liability law for acts or omissions by our pathologists, laboratory personnel and IDN employees who are under our supervision. We are subject to the attendant risk of substantial damages awards in excess of our insurance coverage and risk to our reputation.
We are subject to numerous legal and regulatory requirements governing our activities, and we may face substantial fines and penalties, and our business activities may be impacted, if we fail to comply.
Our business is subject to or impacted by extensive and frequently changing laws and regulations in the United States (including at both the federal and state levels) and the other jurisdictions in which we engage in business. While we seek to conduct our business in compliance with all applicable laws, many of the laws and regulations applicable to us are vague or indefinite and have not been extensively interpreted by the courts, including many of those relating to:
| ● | billing and reimbursement of clinical testing; | |
| ● | certification or licensure of clinical laboratories; | |
| ● | the anti-self-referral and anti-kickback laws and regulations; |
32
| ● | the laws and regulations administered by the FDA; | |
| ● | the corporate practice of medicine; | |
| ● | operational, personnel and quality requirements intended to ensure that clinical testing services are accurate, reliable and timely; | |
| ● | physician fee splitting; | |
| ● | relationships with physicians and IDNs; | |
| ● | marketing to consumers; | |
| ● | privacy of patient data and other personal information; | |
| ● | safety and health of laboratory employees; and | |
| ● | handling, transportation and disposal of medical specimens, infectious and hazardous waste and radioactive materials. |
These laws and regulations may be interpreted or applied by a prosecutorial, regulatory or judicial authority in a manner that could require us to make changes in our operations, including our pricing and/or billing practices. We may not be able to maintain, renew or secure required permits, licenses or any other regulatory approvals needed to operate our business or commercialize our services. If we fail to comply with applicable laws and regulations, or if we fail to maintain, renew or obtain necessary permits, licenses and approvals, we could suffer civil and criminal penalties, fines, exclusion from participation in governmental healthcare programs and the loss of various licenses, certificates and authorizations necessary to operate our business, as well as incur additional liabilities from third-party claims. If any of the foregoing were to occur, our reputation could be damaged and important business relationships with third parties could be adversely affected.
We also are subject from time to time to qui tam claims brought by former employees or other “whistleblowers.” The federal and state governments continue aggressive enforcement efforts against perceived healthcare fraud. Legislative provisions relating to healthcare fraud and abuse provide government enforcement personnel substantial funding, powers, penalties and remedies to pursue suspected cases of fraud and abuse. In addition, the government has substantial leverage in negotiating settlements since the amount of potential damages far exceeds the rates at which we are reimbursed for our services, and the government has the remedy of excluding a non-compliant provider from participation in the Medicare and Medicaid programs. Regardless of merit or eventual outcome, these types of investigations and related litigation can result in:
| ● | diversion of management time and attention; | |
| ● | expenditure of large amounts of cash on legal fees, costs and payment of damages; | |
| ● | increases to our administrative, billing or other operating costs; | |
| ● | limitations on our ability to continue some of our operations; | |
| ● | enforcement actions, fines and penalties or the assertion of private litigation claims and damages; | |
| ● | decreases to the amount of reimbursement related to diagnostic information services performed; | |
| ● | adverse affects to important business relationships with third parties; | |
| ● | decreased demand for our services; and/or | |
| ● | injury to our reputation. |
Changes in applicable laws and regulations may result in existing practices becoming more restricted, or subject our existing or proposed services to additional costs, delay, modification or withdrawal. Such changes also could require us to modify our business objectives.
33
Failure to accurately bill for our services, or to comply with applicable laws relating to government healthcare programs, could have a material adverse effect on our business.
Billing for diagnostic information services is complex and subject to extensive and non-uniform rules and administrative requirements. Depending on the billing arrangement and applicable law, we bill various payers, such as patients, insurance companies, Medicare, Medicaid, clinicians, IDNs and employer groups. The majority of billing and related operations for our Company are being provided by a third party under our oversight. Failure to accurately bill for our services could have a material adverse effect on our business. In addition, failure to comply with applicable laws relating to billing government healthcare programs may result in various consequences, including: civil and criminal fines and penalties, exclusion from participation in governmental healthcare programs and the loss of various licenses, certificates and authorizations necessary to operate our business, as well as incur additional liabilities from third-party claims. Certain violations of these laws may also provide the basis for a civil remedy under the federal False Claims Act, including fines and damages of up to three times the amount claimed. The qui tam provisions of the federal False Claims Act and similar provisions in certain state false claims acts allow private individuals to bring lawsuits against healthcare companies on behalf of government payers, private payers and/or patients alleging inappropriate billing practices.
Although we believe that we are in compliance, in all material respects, with applicable laws and regulations, there can be no assurance that a regulatory agency or tribunal would not reach a different conclusion. The federal or state government may bring claims based on our current practices, which we believe are lawful. The federal and state governments have substantial leverage in negotiating settlements since the amount of potential damages and fines far exceeds the rates at which we are reimbursed, and the government has the remedy of excluding a non-compliant provider from participation in the Medicare and Medicaid programs. We believe that federal and state governments continue aggressive enforcement efforts against perceived healthcare fraud. Legislative provisions relating to healthcare fraud and abuse provide government enforcement personnel with substantial funding, powers, penalties and remedies to pursue suspected cases of fraud and abuse.
Inflationary pressures could adversely impact us because of increases in the costs of materials, supplies and services, and increased labor and people-related expenses.
Inflationary pressures have resulted in increases in the costs of the testing equipment, supplies and other goods and services that we purchase from manufacturers, suppliers and others. Inflationary pressures, along with the competition for labor, have also resulted in a rise of our labor costs, which include the costs of compensation, benefits, and recruiting and training new hires. Our ability to raise the prices and fees we charge for the services we provide is limited. Continuation of the current inflationary environment may adversely impact us.
Risk Factors Related to Clinical and Commercialization Activity
We may not be able to file INDs to commence additional clinical trials on the timelines we expect, and even if we are able to do so, the FDA may not permit us to proceed.
Avalon has initiated its first-in-human clinical trial of CD19 CAR-T candidate, AVA-001 in August 2019 at the Hebei Yanda Lu Daopei Hospital and Beijing Lu Daopei Hospital in China (the world’s single largest CAR-T treatment network with over 600 patients being treated with CAR-T) for the indication of relapsed/refractory B-cell acute lymphoblastic leukemia and non-Hodgkin Lymphoma. We hope to file a number of investigational new drug applications, or INDs, for cell based therapies and diagnostic systems through INDs over the next several years. However, the timing of our filing of these INDs is primarily dependent on receiving further data from our pre-clinical studies, and our timing of filing on all product candidates is subject to further research. Additionally, our submission of INDs is contingent upon having sufficient financial resources to prepare and complete the application.
We cannot be sure that submission of an IND will result in the United States Food and Drug Administration, or FDA, allowing further clinical trials to begin, or that, once begun, issues will not arise that result in the suspension or termination of such clinical trials. Any IND we submit could be denied by the FDA or the FDA could place any future investigation of ours on clinical hold until we provide additional information, either before or after clinical trials are initiated. Additionally, even if such regulatory authorities agree with the design and implementation of the clinical trials set forth in an IND or clinical trial application, we cannot guarantee that such regulatory authorities will not change their requirements in the future. Unfavorable future trial results or other factors, such as insufficient capital to continue development of a product candidate or program, could also cause us to voluntarily withdraw an effective IND.
34
We have limited experience in conducting clinical trials.
We have limited human clinical trial experience with respect to our product candidates. Although our CEO, Dr. David Jin, is formerly with the FDA, this will not provide assurance of success. The clinical testing process is governed by stringent regulation and is highly complex, costly, time-consuming, and uncertain as to outcome, and pharmaceutical products and products used in the regeneration of tissue may invite particularly close scrutiny and requirements from the FDA and other regulatory bodies. Our failure or the failure of our collaborators to conduct human clinical trials successfully or our failure to capitalize on the results of human clinical trials for our product candidates would have a material adverse effect on us. If our clinical trials of our product candidates or future product candidates do not sufficiently enroll or produce results necessary to support regulatory approval in the United States or elsewhere, or if they show undesirable side effects, we will be unable to commercialize these product candidates.
To receive regulatory approval for the commercial sale of our product candidates, we must conduct adequate and well-controlled clinical trials to demonstrate efficacy and safety in humans. Clinical failure can occur at any stage of the testing. Our clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical and/or non-clinical testing. In addition, the results of our clinical trials may show that our product candidates are ineffective or may cause undesirable side effects, which could interrupt, delay or halt clinical trials, resulting in the denial of regulatory approval by the FDA and other regulatory authorities. In addition, negative, delayed or inconclusive results may result in:
| ● | the withdrawal of clinical trial participants; |
| ● | the termination of clinical trial sites or entire trial programs; |
| ● | costs of related litigation; |
| ● | substantial monetary awards to patients or other claimants; |
| ● | impairment of our business reputation; |
| ● | loss of revenues; and |
| ● | the inability to commercialize our product candidates. |
Delays in the commencement, enrollment, and completion of clinical testing could result in increased costs to us and delay or limit our ability to obtain regulatory approval for our product candidates.
Delays in the commencement, enrollment or completion of clinical testing could significantly affect our product development costs. A clinical trial may be suspended or terminated by us, the FDA, or other regulatory authorities due to a number of factors. The commencement and completion of clinical trials require us to identify and maintain a sufficient number of trial sites, many of which may already be engaged in other clinical trial programs for the same indication as our product candidates. We may be required to withdraw from a clinical trial as a result of changing standards of care, or we may become ineligible to participate in clinical studies. We do not know whether planned clinical trials will begin on time or be completed on schedule, if at all. The commencement, enrollment and completion of clinical trials can be delayed for a number of reasons, including, but not limited to, delays related to:
| ● | findings in pre-clinical studies; |
| ● | reaching agreements on acceptable terms with prospective clinical research organizations, or CROs, and trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| ● | obtaining regulatory approval to commence a clinical trial; |
| ● | complying with conditions imposed by a regulatory authority regarding the scope or term of a clinical trial, or being required to conduct additional trials before moving on to the next phase of trials; |
| ● | obtaining institutional review board, or IRB, approval to conduct a clinical trial at numerous prospective sites; |
| ● | recruiting and enrolling patients to participate in clinical trials for a variety of reasons, including the size of the patient population, nature of trial protocol, meeting the enrollment criteria for our studies, screening failures, the inability of the sites to conduct trial procedures properly, the availability of approved effective treatments for the relevant disease and competition from other clinical trial programs for similar indications; |
35
| ● | retaining patients who have initiated their participation in a clinical trial but may be prone to withdraw due to the treatment protocol, lack of efficacy, personal issues, or side effects from the therapy, or who are lost to further follow-up; |
| ● | manufacturing sufficient quantities of a product candidate for use in clinical trials on a timely basis; |
| ● | complying with design protocols of any applicable special protocol assessment we receive from the FDA; |
| ● | severe or unexpected cell therapy side effects experienced by patients in a clinical trial; |
| ● | collecting, analyzing and reporting final data from the clinical trials; |
| ● | breaches in quality of manufacturing runs that compromise all or some of the doses made; positive results in FDA-required viral testing; karyotypic abnormalities in our cell product; or contamination in our manufacturing facilities, all of which events would necessitate disposal of all cells made from that source; |
| ● | availability of materials provided by third parties necessary to manufacture our product candidates; |
| ● | availability of adequate amounts of acceptable tissue for preparation of master cell banks for our products; and |
| ● | requirements to conduct additional trials and studies, and increased expenses associated with the services of our CROs and other third parties. |
If we are required to conduct additional clinical trials or other testing of our product candidates beyond those that we currently contemplate, we or our development partners, if any, may be delayed in obtaining, or may not be able to obtain or maintain, clinical or marketing approval for these product candidates. We may not be able to obtain approval for indications that are as broad as intended, or we may be able to obtain approval only for indications that are entirely different from those indications for which we sought approval.
Changes in regulatory requirements and guidance may occur, and we may need to amend clinical trial protocols to reflect these changes with appropriate regulatory authorities. Amendments may require us to resubmit our clinical trial protocols to IRBs for re-examination, which may impact the costs, timing, or successful completion of a clinical trial. If we experience delays in the completion of, or if we terminate, our clinical trials, the commercial prospects for our product candidates will be harmed, and our ability to generate product revenues will be delayed. In addition, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of a product candidate. Even if we are able to ultimately commercialize our product candidates, other therapies for the same or similar indications may have been introduced to the market and already established a competitive advantage. Any delays in obtaining regulatory approvals may:
| ● | delay commercialization of, and our ability to derive product revenues from, our product candidates; |
| ● | impose costly procedures on us; or |
| ● | diminish any competitive advantages that we may otherwise enjoy. |
36
Our success depends upon the viability of our product candidates and we cannot be certain any of them will receive regulatory approval to be commercialized.
We will need FDA approval to market and sell any of our product candidates in the United States and approvals from FDA-equivalent regulatory authorities in foreign jurisdictions to commercialize our product candidates in those jurisdictions. In order to obtain FDA approval of any of our product candidates, we must submit to the FDA a new drug application, or NDA, or a biologics license application, or BLA, demonstrating that the product candidate is safe for humans and effective for its intended use. This demonstration requires significant research and animal tests, which are referred to as pre-clinical studies, as well as human tests, which are referred to as clinical trials. Satisfaction of the FDA’s regulatory requirements typically takes many years, depends upon the type, complexity, and novelty of the product candidate, and requires substantial resources for research, development, testing and manufacturing. We cannot predict whether our research and clinical approaches will result in cell therapies that the FDA considers safe for humans and effective for indicated uses. The FDA has substantial discretion in the drug approval process and may require us to conduct additional pre-clinical and clinical testing or to perform post-marketing studies. The approval process may also be delayed by changes in government regulation, future legislation, administrative action or changes in FDA policy that occur prior to or during our regulatory review.
Even if we comply with all FDA requests, the FDA may ultimately reject one or more of our NDAs or BLAs, as applicable. We cannot be sure that we will ever obtain regulatory clearance for our product candidates. Failure to obtain FDA approval of any of our product candidates will reduce our number of potentially salable products and, therefore, corresponding product revenues, and will have a material and adverse impact on our business.
As the results of earlier pre-clinical studies or clinical trials are not necessarily predictive of future results, any product candidate we advance into clinical trials may not have favorable results in later clinical trials or receive regulatory approval.
Even if our pre-clinical studies and clinical trials are completed as planned, clinical trials, we cannot be certain that their results will support the claims of our product candidates. Positive results in pre-clinical testing and early clinical trials do not ensure that results from later clinical trials will also be positive, and we cannot be sure that the results of later clinical trials will replicate the results of prior clinical trials and pre-clinical testing. A number of companies in the pharmaceutical industry, including those with greater resources and experience, have suffered significant setbacks in Phase II or Phase III clinical trials, even after seeing promising results in earlier clinical trials.
Our clinical trial process may fail to demonstrate that our product candidates are safe for humans and effective for indicated uses. This failure would cause us to abandon a product candidate and may delay development of other product candidates. Any delay in, or termination of, our clinical trials will delay or cause us to refrain from the filing of our NDAs and/or BLAs with the FDA and, ultimately, our ability to commercialize our product candidates and generate product revenues. In addition, our clinical trials to date involve small patient populations. Because of the small sample size, the results of these clinical trials may not be indicative of future results.
Our business faces significant government regulation, and there is no guarantee that our product candidates will receive regulatory approval.
Our research and development activities, pre-clinical studies, anticipated human clinical trials, and anticipated manufacturing and marketing of our potential products are subject to extensive regulation by the FDA and other regulatory authorities in the United States, as well as by regulatory authorities in other countries. In the United States, our product candidates are subject to regulation as biological products or as combination biological products/medical devices under the Federal Food, Drug and Cosmetic Act, the Public Health Service Act and other statutes, as outlined in the Code of Federal Regulations. Different regulatory requirements may apply to our products depending on how they are categorized by the FDA under these laws. These regulations can be subject to substantial and significant interpretation, addition, amendment or revision by the FDA and by the legislative process. The FDA may determine that we will need to undertake clinical trials beyond those currently planned. Furthermore, the FDA may determine that results of clinical trials do not support approval for the product. Similar determinations may be encountered in foreign countries. The FDA will continue to monitor products in the market after approval, if any, and may determine to withdraw its approval or otherwise seriously affect the marketing efforts for any such product. The same possibilities exist for trials to be conducted outside of the United States that are subject to regulations established by local authorities and local law. Any such determinations would delay or deny the introduction of our product candidates to the market and have a material adverse effect on our business, financial condition, and results of operations.
37
Cell based therapeutics are subject to ongoing periodic unannounced inspection by the FDA, the Drug Enforcement Agency, other federal agencies and corresponding state agencies to ensure strict compliance with good manufacturing practices, and other government regulations and corresponding foreign standards. We do not have control over third-party manufacturers’ compliance with these regulations and standards, nor can we guarantee that we will maintain compliance with such regulations in regards to our own manufacturing processes. Other risks include:
| ● | regulatory authorities may require the addition of labeling statements, specific warnings, a contraindication, or field alerts to physicians and pharmacies; |
| ● | regulatory authorities may withdraw their approval of the IND or the product or require us to take our approved products off the market; |
| ● | we may be required to change the way the product is manufactured or administered and we may be required to conduct additional clinical trials or change the labeling of our products; |
| ● | we may have limitations on how we promote our products; and |
| ● | we may be subject to litigation or product liability claims. |
Even if our product candidates receive regulatory approval in the United States, we may never receive approval or commercialize our product candidates outside of the United States. In order to market and commercialize any product candidate outside of the United States, we must establish and comply with numerous and varying regulatory requirements of other countries regarding manufacturing, safety and efficacy. Approval procedures vary among countries and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries might differ from that required to obtain FDA approval. The regulatory approval process in other countries may include all of the risks detailed above regarding FDA approval in the United States as well as other risks. Regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory approval process in others. Failure to obtain regulatory approval in other countries, or any delay or setback in obtaining such approval, could have the same adverse effects detailed above regarding FDA approval in the United States. Such effects include the risks that our product candidates may not be approved for all indications requested, which could limit the uses of our product candidates and have an adverse effect on product sales and potential royalties, and that such approval may be subject to limitations on the indicated uses for which the product may be marketed or require costly, post-marketing follow-up studies.
Even if our product candidates receive regulatory approval, we may still face future development and regulatory difficulties.
Even if U.S. regulatory approval is obtained, the FDA may still impose significant restrictions on a product’s indicated uses or marketing, or impose ongoing requirements for potentially costly post-approval studies. If any of our products were granted accelerated approval, FDA could require post-marketing confirmatory trials to verify and describe the anticipated effect on irreversible morbidity or mortality or other clinical benefit. FDA may withdraw approval of a drug or indication approved under the accelerated approval pathway if a trial required to verify the predicted clinical benefit of the product fails to verify such benefit; other evidence demonstrates that the product is not shown to be safe or effective under the conditions of use; the applicant fails to conduct any required post-approval trial of the drug with due diligence; or the applicant disseminates false or misleading promotional materials relating to the product. In addition, the FDA currently requires as a condition for accelerated approval the pre-approval of promotional materials, which could adversely impact the timing of the commercial launch of the product.
Given the number of recent high-profile adverse safety events with certain drug and cell related products, the FDA may require, as a condition of approval, costly risk management programs, which may include safety surveillance, restricted distribution and use, patient education, enhanced labeling, special packaging or labeling, expedited reporting of certain adverse events, pre-approval of promotional materials, and restrictions on direct-to-consumer advertising. Furthermore, heightened Congressional scrutiny on the adequacy of the FDA’s drug approval process and the FDA’s efforts to assure the safety of marketed cell based therapy has resulted in the proposal of new legislation addressing drug safety issues. If enacted, any new legislation could result in delays or increased costs during the period of product development, clinical trials, and regulatory review and approval, as well as increased costs to assure compliance with any new post-approval regulatory requirements. Any of these restrictions or requirements could force us to conduct costly studies or increase the time for us to become profitable. For example, any labeling approved for any of our product candidates may include a restriction on the term of its use, or it may not include one or more of our intended indications.
38
Our product candidates will also be subject to ongoing FDA requirements for the labeling, packaging, storage, advertising, promotion, record-keeping, and submission of safety and other post-market information on the cell based therapy. New issues may arise during a product lifecycle that did not exist, or were unknown, at the time of product approval, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured. Since approved products, manufacturers, and manufacturers’ facilities are subject to continuous review and periodic inspections, these new issues post-approval may result in voluntary actions by us or may result in a regulatory agency imposing restrictions on that product or us, including requiring withdrawal of the product from the market or for use in a clinical study. If our product candidates fail to comply with applicable regulatory requirements, such as good manufacturing practices, a regulatory agency may:
| ● | issue warning letters; |
| ● | require us to enter into a consent decree, which can include imposition of various fines, reimbursements for inspection costs, required due dates for specific actions, and penalties for noncompliance; |
| ● | impose other civil or criminal penalties; |
| ● | suspend regulatory approval; |
| ● | suspend any ongoing clinical trials; |
| ● | refuse to approve pending applications or supplements to approved applications filed by us; |
| ● | impose restrictions on operations, including costly new manufacturing requirements; or |
| ● | seize or detain products or require a product recall. |
If we or current or future collaborators, manufacturers, or service providers fail to comply with healthcare laws and regulations, we or they could be subject to enforcement actions and substantial penalties, which could affect our ability to develop, market and sell our products and may harm our reputation.
Although we do not currently have any products on the market, once our therapeutic candidates or clinical trials are covered by federal health care programs, we will be subject to additional healthcare statutory and regulatory requirements and enforcement by the federal, state and foreign governments of the jurisdictions in which we conduct our business. Healthcare providers, physicians and third party payors play a primary role in the recommendation and prescription of any therapeutic candidates for which we obtain marketing approval. Our future arrangements with third party payors and customers may expose us to broadly applicable fraud and abuse, transparency, and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our therapeutic candidates for which we obtain marketing approval. Restrictions under applicable federal and state healthcare laws and regulations include, but are not limited to, the following:
| ● | the U.S. federal Anti-Kickback Statute, which prohibits, among other things, persons from soliciting, receiving, offering or providing remuneration, directly or indirectly, to induce either the referral of an individual for a healthcare item or service, or the purchasing or ordering of an item or service, for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare or Medicaid; |
| ● | federal civil and criminal false claims laws and civil monetary penalty laws, such as the U.S. federal FCA, which imposes criminal and civil penalties, including through civil whistleblower or qui tam actions, against, individuals or entities for knowingly presenting or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government. In addition, the government may assert that a claim including items and services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the FCA; |
39
| ● | HIPAA includes a fraud and abuse provision referred to as the HIPAA All-Payor Fraud Law, which imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program, or knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation; |
| ● | HIPAA, as amended by HITECH, and its implementing regulations, which impose obligations on certain covered entity healthcare providers, health plans, and healthcare clearinghouses as well as their business associates that perform certain services involving the use or disclosure of individually identifiable health information, including mandatory contractual terms, with respect to safeguarding, the privacy, security, and transmission of individually identifiable health information, and require notification to affected individuals and regulatory authorities of certain breaches of security of individually identifiable health information; |
| ● | federal and state consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers; |
| ● | the federal Physician Payment Sunshine Act and the implementing regulations, also referred to as “Open Payments,” issued under the ACA, which require that manufacturers of pharmaceutical and biological drugs reimbursable under Medicare, Medicaid, and Children’s Health Insurance Programs report to the Department of Health and Human Services all consulting fees, travel reimbursements, research grants, and other payments, transfers of value or gifts made to physicians and teaching hospitals with limited exceptions; and |
| ● | analogous state laws and regulations, such as, state anti-kickback and false claims laws potentially applicable to sales or marketing arrangements and claims involving healthcare items or services reimbursed by nongovernmental third party payors, including private insurers; and some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug and cell based therapy manufacturers to report information related to payments to physicians and other healthcare providers or marketing expenditures, and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
The scope and enforcement of each of these laws is uncertain and subject to rapid change in the current environment of healthcare reform, especially in light of the lack of applicable precedent and regulations. Federal and state enforcement bodies have recently increased their scrutiny of interactions between healthcare companies and healthcare providers, which has led to a number of investigations, prosecutions, convictions and settlements in the healthcare industry. Responding to investigations can be time-and resource-consuming and can divert management’s attention from the business. Any such investigation or settlement could increase our costs or otherwise have an adverse effect on our business.
Ensuring that our business arrangements with third-parties comply with applicable healthcare laws and regulations could involve substantial costs. If our operations are found to be in violation of any such requirements, we may be subject to penalties, including civil or criminal penalties, monetary damages, the curtailment or restructuring of our operations, or exclusion from participation in government contracting, healthcare reimbursement or other government programs, including Medicare and Medicaid, any of which could adversely affect our financial results. Although effective compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, these risks cannot be entirely eliminated. Any action against us for an alleged or suspected violation could cause us to incur significant legal expenses and could divert our management’s attention from the operation of our business, even if our defense is successful. In addition, achieving and sustaining compliance with applicable laws and regulations may be costly to us in terms of money, time and resources.
40
Any cell based therapies we develop may become subject to unfavorable pricing regulations, third party coverage and reimbursement practices or healthcare reform initiatives, thereby harming our business.
The regulations that govern marketing approvals, pricing, coverage and reimbursement for new drugs and cell based therapies vary widely from country to country. Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. Although we intend to monitor these regulations, our programs are currently in earlier stages of development and we will not be able to assess the impact of price regulations for a number of years. As a result, we might obtain regulatory approval for a product in a particular country, but then be subject to price regulations that delay our commercial launch of the product and negatively impact the revenues we are able to generate from the sale of the product in that country.
Our ability to commercialize any products successfully also will depend in part on the extent to which coverage and reimbursement for these products and related treatments will be available from government health administration authorities, private health insurers and other organizations. However, there may be significant delays in obtaining coverage for newly-approved cell based therapies. Moreover, eligibility for coverage does not necessarily signify that a cell based therapy will be reimbursed in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution costs. Also, interim payments for new cell based therapy if applicable, may be insufficient to cover our costs and may not be made permanent. Thus, even if we succeed in bringing one or more products to the market, these products may not be considered medically necessary or cost-effective, and the amount reimbursed for any products may be insufficient to allow us to sell our products on a competitive basis. Because our programs are in earlier stages of development, we are unable at this time to determine their cost effectiveness, or the likely level or method of reimbursement. In addition, obtaining coverage and reimbursement approval of a product from a government or other third-party payor is a time-consuming and costly process that could require us to provide to each payor supporting scientific, clinical and cost-effectiveness data for the use of our product on a payor-by-payor basis, with no assurance that coverage and adequate reimbursement will be obtained. A payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved. Further, one payor’s determination to provide coverage for a product does not assure that other payors will also provide coverage for the product. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development. If reimbursement is not available or is available only at limited levels, we may not be able to successfully commercialize any product candidate that we successfully develop.
Increasingly, the third party payors who reimburse patients or healthcare providers, such as government and private insurance plans, are seeking greater upfront discounts, additional rebates and other concessions to reduce the prices for pharmaceutical products. If the price we are able to charge for any products we develop, or the reimbursement provided for such products, is inadequate in light of our development and other costs, our return on investment could be adversely affected.
We currently expect that certain drugs we develop may need to be administered under the supervision of a physician on an outpatient basis. Under currently applicable U.S. law, certain drugs that are not usually self-administered (including injectable cell based therapies) may be eligible for coverage under Medicare through Medicare Part B. Specifically, Medicare Part B coverage may be available for eligible beneficiaries when the following, among other requirements have been satisfied:
| ● | the product is reasonable and necessary for the diagnosis or treatment of the illness or injury for which the product is administered according to accepted standards of medical practice; |
| ● | the product is typically furnished incident to a physician’s services; |
| ● | the indication for which the product will be used is included or approved for inclusion in certain Medicare-designated pharmaceutical compendia (when used for an off-label use); and |
| ● | the product has been approved by the FDA. |
41
Average prices for cell therapies may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs and cell based therapy from countries where they may be sold at lower prices than in the U.S. Reimbursement rates under Medicare Part B would depend in part on whether the newly approved product would be eligible for a unique billing code. Self-administered, outpatient drugs and cell based therapies are typically reimbursed under Medicare Part D, and cell based therapies that are administered in an inpatient hospital setting are typically reimbursed under Medicare Part A under a bundled payment. It is difficult for us to predict how Medicare coverage and reimbursement policies will be applied to our products in the future and coverage and reimbursement under different federal healthcare programs are not always consistent. Medicare reimbursement rates may also reflect budgetary constraints placed on the Medicare program.
Third party payors often rely upon Medicare coverage policies and payment limitations in setting their own reimbursement rates. These coverage policies and limitations may rely, in part, on compendia listings for approved therapeutics. Our inability to promptly obtain relevant compendia listings, coverage, and adequate reimbursement from both government-funded and private payors for new cell based therapies that we develop and for which we obtain regulatory approval could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products and our financial condition.
We expect that these and other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and lower reimbursement, and in additional downward pressure on the price that we receive for any approved product. Any reduction in reimbursement from Medicare or other government-funded programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our cell based therapies, once marketing approval is obtained.
We believe that the efforts of governments and third party payors to contain or reduce the cost of healthcare and legislative and regulatory proposals to broaden the availability of healthcare will continue to affect the business and financial condition of pharmaceutical and biopharmaceutical companies. A number of legislative and regulatory changes in the healthcare system in the U.S. and other major healthcare markets have been proposed, and such efforts have expanded substantially in recent years. These developments could, directly or indirectly, affect our ability to sell our products, if approved, at a favorable price. For example, in the United States, in 2010, the U.S. Congress passed the ACA, a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of health spending, enhance remedies against fraud and abuse, add new transparency requirements for the healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional policy reforms. Among the provisions of the ACA addressing coverage and reimbursement of pharmaceutical products, of importance to our potential therapeutic candidates are the following:
| ● | increases to pharmaceutical manufacturer rebate liability under the Medicaid Drug Rebate Program due to an increase in the minimum basic Medicaid rebate on most branded prescription drugs and the application of Medicaid rebate liability to drugs used in risk-based Medicaid managed care plans; |
| ● | the expansion of the 340B Drug Pricing Program to require discounts for “covered outpatient drugs” sold to certain children’s hospitals, critical access hospitals, freestanding cancer hospitals, rural referral centers, and sole community hospitals; |
| ● | requirements imposed on pharmaceutical companies are required to offer discounts on brand-name cell based therapy to patients who fall within the Medicare Part D coverage gap, commonly referred to as the “Donut Hole”; |
| ● | requirements imposed on pharmaceutical companies to pay an annual non-tax-deductible fee to the federal government based on each company’s market share of prior year total sales of branded drugs to certain federal healthcare programs, such as Medicare, Medicaid, Department of Veterans Affairs and Department of Defense; and |
42
| ● | for products classified as biologics, marketing approval for a follow-on biologic product may not become effective until 12 years after the date on which the reference innovator biologic product was first licensed by the FDA, with a possible six-month extension for pediatric products. After this exclusivity ends, it may be possible for biosimilar manufacturers to enter the market, which is likely to reduce the pricing for the innovator product and could affect our profitability if our products are classified as biologics. |
Separately, pursuant to the health reform legislation and related initiatives, the Centers for Medicare and Medicaid Services, or CMS, is working with various healthcare providers to develop, refine, and implement Accountable Care Organizations, or ACOs, and other innovative models of care for Medicare and Medicaid beneficiaries, including the Bundled Payments for Care Improvement Initiative, the Comprehensive Primary Care Initiative, the Duals Demonstration, and other models. The continued development and expansion of ACOs and other innovative models of care will have an uncertain impact on any future reimbursement we may receive for approved therapeutics administered by these organizations.
The healthcare industry is heavily regulated in the U.S. at the federal, state, and local levels, and our failure to comply with applicable requirements may subject us to penalties and negatively affect our financial condition.
As a healthcare company, our operations, clinical trial activities and interactions with healthcare providers may be subject to extensive regulation in the U.S., particularly if we receive FDA approval for any of its products in the future. For example, if we receive FDA approval for a product for which reimbursement is available under a federal healthcare program (e.g., Medicare, Medicaid), it would be subject to a variety of federal laws and regulations, including those that prohibit the filing of false or improper claims for payment by federal healthcare programs (e.g. the federal False Claims Act), prohibit unlawful inducements for the referral of business reimbursable by federal healthcare programs (e.g. the federal Anti-Kickback Statute), and require disclosure of certain payments or other transfers of value made to U.S.-licensed physicians and teaching hospitals or Open Payments. We are not able to predict how third parties will interpret these laws and apply applicable governmental guidance and may challenge our practices and activities under one or more of these laws. If our past or present operations are found to be in violation of any of these laws, we could be subject to civil and criminal penalties, which could hurt our business, our operations and financial condition.
The federal Anti-Kickback Statute prohibits, among other things, any person or entity, from knowingly and willfully offering, paying, soliciting or receiving any remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce or in return for purchasing, leasing, ordering or arranging for the purchase, lease or order of any item or service reimbursable under Medicare, Medicaid or other federal healthcare programs. The term remuneration has been interpreted broadly to include anything of value. The Anti-Kickback Statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on one hand and prescribers, purchasers, and formulary managers on the other. There are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution. The exceptions and safe harbors are drawn narrowly and practices that involve remuneration that may be alleged to be intended to induce prescribing, purchasing or recommending may be subject to scrutiny if they do not qualify for an exception or safe harbor. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the Anti-Kickback Statute. Instead, the legality of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all of its facts and circumstances. Our practices may not in all cases meet all of the criteria for protection under a statutory exception or regulatory safe harbor.
Additionally, the intent standard under the Anti-Kickback Statute was amended by the ACA, to a stricter standard such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. In addition, the ACA codified case law that a claim including items or services resulting from a violation of the federal Anti- Kickback Statute constitutes a false or fraudulent claim for purposes of the federal FCA.
The civil monetary penalties statute imposes penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal healthcare program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent.
43
Federal false claims and false statement laws, including the federal FCA, prohibit, among other things, any person or entity from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment to, or approval by, the federal healthcare programs, including Medicare and Medicaid, or knowingly making, using, or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government. A claim includes “any request or demand” for money or property presented to the U.S. government. For instance, historically, pharmaceutical and other healthcare companies have been prosecuted under these laws for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. Other companies have been prosecuted for causing false claims to be submitted because of the companies’ marketing of the product for unapproved, off-label, and thus generally non-reimbursable, uses.
HIPAA prohibits, among other offenses, knowingly and willfully executing a scheme to defraud any health care benefit program, including private payors, or falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for items or services under a health care benefit program. To the extent that we act as a business associate to a healthcare provider engaging in electronic transactions, we may also be subject to the privacy and security provisions of HIPAA, as amended by HITECH, which restricts the use and disclosure of patient-identifiable health information, mandates the adoption of standards relating to the privacy and security of patient-identifiable health information, and requires the reporting of certain security breaches to healthcare provider customers with respect to such information. Additionally, many states have enacted similar laws that may impose more stringent requirements on entities like ours. Failure to comply with applicable laws and regulations could result in substantial penalties and adversely affect our financial condition and results of operations.
Many states also have similar fraud and abuse statutes or regulations that apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. Additionally, to the extent that our product is sold in a foreign country, we may be subject to similar foreign laws.
Our products, once approved, may be eligible for coverage under Medicare and Medicaid, among other government healthcare programs. Accordingly, we may be subject to a number of obligations based on their participation in these programs, such as a requirement to calculate and report certain price reporting metrics to the government, such as average sales price (ASP) and best price. Penalties may apply in some cases when such metrics are not submitted accurately and timely. Further, these prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs and biological products from countries where they may be sold at lower prices than in the United States. It is difficult to predict how Medicare coverage and reimbursement policies will be applied to our products in the future and coverage and reimbursement under different federal healthcare programs are not always consistent. Medicare reimbursement rates may also reflect budgetary constraints placed on the Medicare program.
In order to distribute products commercially, we must comply with state laws that require the registration of manufacturers and wholesale distributors of drug and biological products in a state, including, in certain states, manufacturers and distributors who ship products into the state even if such manufacturers or distributors have no place of business within the state. Some states also impose requirements on manufacturers and distributors to establish the pedigree of product in the chain of distribution, including some states that require manufacturers and others to adopt new technology capable of tracking and tracing product as it moves through the distribution chain. Several states have enacted legislation requiring pharmaceutical and biotechnology companies to establish marketing compliance programs, file periodic reports with the state, make periodic public disclosures on sales, marketing, pricing, clinical trials and other activities, and/or register their sales representatives, as well as to prohibit pharmacies and other healthcare entities from providing certain physician prescribing data to pharmaceutical and biotechnology companies for use in sales and marketing, and to prohibit certain other sales and marketing practices. All of our activities are potentially subject to federal and state consumer protection and unfair competition laws.
If our operations are found to be in violation of any of the federal and state healthcare laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including without limitation, civil, criminal and/or administrative penalties, damages, fines, disgorgement, exclusion from participation in government programs, such as Medicare and Medicaid, injunctions, private “qui tam” actions brought by individual whistleblowers in the name of the government, or refusal to allow us to enter into government contracts, contractual damages, reputational harm, administrative burdens, diminished profits and future earnings, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
44
Our ability to obtain reimbursement or funding from the federal government may be impacted by possible reductions in federal spending.
U.S. federal government agencies currently face potentially significant spending reductions. The Budget Control Act of 2011, or the BCA, established a Joint Select Committee on Deficit Reduction, which was tasked with achieving a reduction in the federal debt level of at least $1.2 trillion. That committee did not draft a proposal by the BCA’s deadline. As a result, automatic cuts, referred to as sequestration, in various federal programs were scheduled to take place, beginning in January 2013, although the American Taxpayer Relief Act of 2012 delayed the BCA’s automatic cuts until March 1, 2013. While the Medicare program’s eligibility and scope of benefits are generally exempt from these cuts, Medicare payments to providers and Part D health plans are not exempt. The BCA did, however, provide that the Medicare cuts to providers and Part D health plans would not exceed two percent. President Obama issued the sequestration order on March 1, 2013, and cuts went into effect on April 1, 2013. Additionally, the Bipartisan Budget Act of 2015 extended sequestration for Medicare through fiscal year 2027.
The U.S. federal budget remains in flux, which could, among other things, cut Medicare payments to providers. The Medicare program is frequently mentioned as a target for spending cuts. The full impact on our business of any future cuts in Medicare or other programs is uncertain. In addition, we cannot predict any impact President Trump’s administration and the U.S. Congress may have on the federal budget. If federal spending is reduced, anticipated budgetary shortfalls may also impact the ability of relevant agencies, such as the FDA or the National Institutes of Health, to continue to function at current levels. Amounts allocated to federal grants and contracts may be reduced or eliminated. These reductions may also impact the ability of relevant agencies to timely review and approve drug research and development, manufacturing, and marketing activities, which may delay our ability to develop, market and sell any products we may develop.
Risks Related to Our Securities
Our officers, directors and principal stockholders own a significant percentage of our stock and will be able to exert significant control over matters subject to stockholder approval.
Our officers, directors and 5% stockholders and their affiliates beneficially own a significant percentage of our outstanding common stock. As a result, these stockholders have significant influence and may be able to determine all matters requiring stockholder approval. For example, these stockholders may be able to control elections of directors, amendments of our organizational documents, or approval of any merger, sale of assets, or other major corporate transactions. This concentration of ownership could delay or prevent any acquisition of our company on terms that other stockholders may desire, and may adversely affect the market price of our common stock.
If we are unable to maintain listing of our securities on the Nasdaq Capital Market or another reputable stock exchange, it may be more difficult for our stockholders to sell their securities.
Nasdaq requires listing issuers to comply with certain standards in order to remain listed on its exchange. If, for any reason, Nasdaq should delist our securities from trading on its exchange and we are unable to obtain listing on another reputable national securities exchange, a reduction in some or all of the following may occur, each of which could materially adversely affect our stockholders. A delisting of our common stock is likely to reduce the liquidity of our common stock and may inhibit or preclude our ability to raise additional financing.
On February 9, 2022, the Company received notice from The Nasdaq Stock Market (“Nasdaq”) that the closing bid price for the Company’s common stock had been below $1.00 per share for the previous 30 consecutive business days, and that the Company was therefore not in compliance with the minimum bid price requirement for continued inclusion on The Nasdaq Capital Market under Nasdaq Listing Rule 5550(a)(2) (the “Rule”). Nasdaq’s notice had no immediate effect on the listing or trading of the Company’s common stock on The Nasdaq Capital Market. The notice indicated that the Company will have 180 calendar days, until August 8, 2022, to regain compliance with the Rule. On August 9, 2022, we were provided an additional compliance period of 180 calendar days, or until February 6, 2023, to regain compliance with the minimum closing bid requirement. The Company could regain compliance with the $1.00 minimum bid listing requirement if the closing bid price of its common stock is at least $1.00 per share for a minimum of ten (10) consecutive business days during the 180-day compliance period. If the Company did not regain compliance during the initial compliance period, it may be eligible for additional time to regain compliance with the Rule. To qualify, the Company was required to meet the continued listing requirement for market value of its publicly held shares and all other Nasdaq initial listing standards, except the bid price requirement, and provide written notice to Nasdaq of its intention to cure the deficiency during the second compliance period by effecting a reverse stock split, if necessary. If the Company was not eligible or it appeared to Nasdaq that the Company was not be able to cure the deficiency during the second compliance period, Nasdaq then provides written notice to the Company that the Company’s common stock will be subject to delisting. In the event of such notification, the Company may appeal Nasdaq’s determination to delist its securities, but there can be no assurance that Nasdaq would grant the Company’s request for continued listing. We effected a one-for-ten reverse stock split on January 5, 2023. On January 20, 2023, we received a letter from the staff of Nasdaq indicating that we have regained compliance with the Rule and as of the date of this filing, this matter is now closed.
45
The price of our common stock may be volatile and fluctuate substantially, which could result in substantial losses for our stockholders.
Our common stock has been listed on the Nasdaq Capital Market under the symbol “ALBT” since November 10, 2022. Our common stock was listed on the Nasdaq Capital Market under the symbol “AVCO” since November 5, 2018 through the close of business on November 9, 2022. Our common shares were traded previously on the OTC Market Group Inc.’s Venture Market (the “OTCQB”) since February 22, 2016, under the symbol “AVCO” since October 18, 2016 and “GTHC” prior to October 18, 2016.
The price of our common stock has been, and we expect it to continue to be, volatile. The stock market in general and the market for smaller healthcare companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, you may not be able to sell your shares of common stock at or above the price you paid for your shares of common stock. The market price for our common stock may be influenced by many factors, including:
| ● | the success of competitive products or technologies; |
| ● | developments related to our existing or any future collaborations; |
| ● | regulatory or legal developments in the United States and other countries; |
| ● | developments or disputes concerning patent applications, issued patents or other proprietary rights; |
| ● | the recruitment or departure of key personnel; |
| ● | actual or anticipated changes in estimates as to financial results or recommendations by securities analysts; |
| ● | variations in our financial results or those of companies that are perceived to be similar to us; |
| ● | changes in the structure of healthcare payment systems; |
| ● | market conditions in the healthcare, pharmaceutical and biotechnology sectors; |
| ● | general economic, industry and market conditions; and |
| ● | the other factors described in this “Risk Factors” section. |
Future sales of our common stock or securities convertible or exchangeable for our common stock may cause our stock price to decline.
If our existing stockholders sell, or indicate an intention to sell, substantial amounts of our common stock in the public market, the price of our common stock could decline. The perception in the market that these sales may occur could also cause the price of our common stock to decline.
46
In addition, as of December 31, 2022, 924,464 shares of common stock issuable upon exercise of outstanding stock options and warrants and 900,000 shares of common stock issuable upon conversion of outstanding Series A convertible preferred stock, which will become eligible for sale in the public market to the extent permitted by the provisions of various vesting schedules, the lock-up agreements and Rule 144 under the Securities Act. If the shares we may issue from time to time upon exercise of outstanding options and warrants and conversion of outstanding Series A convertible preferred stock are sold, or if it is perceived that they will be sold, by the award recipients in the public market, the price of our common stock could decline.
You may experience dilution of your ownership interests because of the future issuance of additional shares of our common or preferred stock or other securities that are convertible into or exercisable for our common or preferred stock.
As of the date of this filing, we have issued an aggregate of (i) 9,000 shares of our newly designated Series A Convertible Preferred Stock and (ii) 11,000 shares of our newly designated Series B Convertible Preferred Stock. In the future, we may issue our authorized but previously unissued equity securities, resulting in the dilution of the ownership interests of our stockholders. We are authorized to issue an aggregate of 490,000,000 shares of common stock and 10,000,000 shares of “blank check” preferred stock. We may issue additional shares of our common stock or other securities that are convertible into or exercisable for our common stock in connection with hiring or retaining employees, future acquisitions, future sales of our securities for capital raising purposes, or for other business purposes. The future issuance of any such additional shares of our common stock may create downward pressure on the trading price of the common stock. We expect we will need to raise additional capital in the near future to meet our working capital needs, and there can be no assurance that we will not be required to issue additional shares, warrants or other convertible securities in the future in conjunction with these capital raising efforts, including at a price (or exercise prices) below the price you paid for your stock.
The ability of our Board of Directors to issue additional stock may prevent or make more difficult certain transactions, including a sale or merger.
Our Board of Directors is authorized to issue up to 10,000,000 shares of preferred stock with powers, rights and preferences designated by it. Shares of voting or convertible preferred stock could be issued, or rights to purchase such shares could be issued, to create voting impediments or to frustrate persons seeking to effect a takeover or otherwise gain control of us. The rights of holders of our common stock are subject to the rights of the holders of our preferred stock, including our newly designated Series A Convertible Preferred Stock and Series B Convertible Preferred Stock, and any preferred stock that may be issued. The ability of the Board of Directors to issue such additional shares of preferred stock, with rights and preferences it deems advisable, could discourage an attempt by a party to acquire control of us by tender offer or other means. Such issuances could therefore deprive stockholders of benefits that could result from such an attempt, such as the realization of a premium over the market price for their shares in a tender offer or the temporary increase in market price that such an attempt could cause. Moreover, the issuance of such additional shares of preferred stock to persons friendly to the Board of Directors could make it more difficult to remove incumbent managers and directors from office even if such change were to be favorable to stockholders generally.
We are incorporated in Delaware. Certain anti-takeover provisions of Delaware law and our charter documents as currently in effect may make a change in control of us more difficult, even if a change in control would be beneficial to the stockholders. Delaware law also prohibits corporations from engaging in a business combination with any holders of 15% or more of their capital stock until the holder has held the stock for three years unless, among other possibilities, our Board of Directors approves the transaction. Our Board of Directors may use these provisions to prevent changes in the management and control of us. Also, under applicable Delaware law, our Board of Directors may adopt additional anti-takeover measures in the future.
47
If securities or industry analysts do not publish research or reports about our business, or if they issue an adverse or misleading opinion regarding our stock, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts publish about us or our business. We do not currently have and may never obtain research coverage by securities and industry analysts. If no or few securities or industry analysts commence coverage of us, the trading price for our stock would be negatively impacted. In the event we obtain securities or industry analyst coverage, if any of the analysts who cover us issue an adverse or misleading opinion regarding us, our business model, our intellectual property or our stock performance, or if our operating results fail to meet the expectations of analysts, our stock price would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
We do not anticipate paying dividends on our common stock, and investors may lose the entire amount of their investment.
We have never declared or paid cash dividends on our common stock, and we do not anticipate such a declaration or payment for the foreseeable future.
We expect to use future earnings, if any, to fund business growth. Therefore, stockholders will not receive any funds absent a sale of their shares of common stock. We cannot assure stockholders of a positive return on their investment when they sell their shares, nor can we assure that stockholders will not lose the entire amount of their investment.
Applicable regulatory requirements, including those contained in and issued under the Sarbanes-Oxley Act of 2002, may make it difficult for us to retain or attract qualified officers and directors, which could adversely affect the management of our business and our ability to obtain or retain listing of our common stock on a national securities exchange.
We may be unable to attract and retain those qualified officers, directors and members of board committees required to provide for effective management because of the rules and regulations that govern publicly held companies, including, but not limited to, certifications by principal executive officers. The enactment of the Sarbanes-Oxley Act has resulted in the issuance of a series of related rules and regulations and the strengthening of existing rules and regulations by the SEC, as well as the adoption of new and more stringent rules by national securities exchanges. The perceived increased personal risk associated with these changes may deter qualified individuals from accepting roles as directors and executive officers.
48
Further, some of these changes heighten the requirements for board or committee membership, particularly with respect to an individual’s independence from the corporation and level of experience in finance and accounting matters. We may have difficulty attracting and retaining directors with the requisite qualifications. If we are unable to attract and retain qualified officers and directors, the management of our business and our ability to obtain or retain listing of our shares of common stock on any national securities exchange could be adversely affected.
If we cannot satisfy, or continue to satisfy, the initial listing requirements and other rules of the Nasdaq Capital Market, our securities may be delisted, which could negatively impact the price of our securities and your ability to sell them.
Our common stock has been listed on the Nasdaq Capital Market under the symbol “ALBT” since November 10, 2022 and under the symbol “AVCO” since November 5, 2018 through the close of business on November 9, 2022. In order to maintain our listing on the Nasdaq Capital Market, we are required to comply with certain rules of the applicable trading market, including those regarding minimum stockholders’ equity, minimum share price and certain corporate governance requirements. We may not be able to continue to satisfy the listing requirements and other applicable rules of the Nasdaq Capital Market. If we are unable to satisfy the criteria for maintaining our listing, our securities could be subject to delisting.
If our common stock is delisted from trading by the applicable trading market we could face significant consequences, including.
| ● | a limited availability for market quotations for our securities; |
| ● | reduced liquidity with respect to our securities; |
| ● | a determination that our common stock is a “penny stock,” which will require brokers trading in our common stock to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for our common stock; |
| ● | limited amount of news and analyst coverage; and |
| ● | a decreased ability to issue additional securities or obtain additional financing in the future. |
We could be subject to securities class action litigation.
In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because companies in our industry have experienced significant stock price volatility in recent years. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
49
ITEM 2. PROPERTIES
Our principal offices are located at 4400 Route 9 South, Freehold, NJ 07728. The office building is owned by our subsidiary, Avalon RT 9 Properties, LLC, which is in business of owning and operating an income-producing real property. Our property is well maintained, adequately meets our needs, and is being utilized for its intended purpose.
We lease additional office space for operations. Office location is not crucial to our operations, and we anticipate no difficulty in extending these leases or obtaining comparable office space.
We are obligated under various lease agreements providing for office space that expire at various dates through the year 2025. Total rent expense under these lease agreements was approximately $141,000 and $143,000 for the years ended December 31, 2022 and 2021, respectively.
We believe that our current office space is adequate for our current and immediately foreseeable operating needs.
ITEM 3. LEGAL PROCEEDINGS
From time to time, we are subject to ordinary routine litigation incidental to our normal business operations. We are not currently a party to, and our property is not subject to, any material legal proceedings, except as set forth below.
On October 25, 2017, our subsidiary, Genexosome Technologies Inc. (“Genexosome”), entered into and closed a Stock Purchase Agreement with Beijing Jieteng (Genexosome) Biotech Co., Ltd., a corporation incorporated in the People’s Republic of China on August 7, 2015 (“Beijing Genexosome”) which was dissolved in June 2022, and Yu Zhou, MD, PhD, the sole shareholder of Beijing Genexosome, pursuant to which Genexosome acquired all of the issued and outstanding securities of Beijing Genexosome in consideration of a cash payment in the amount of $450,000, of which $100,000 is still owed. Further, on October 25, 2017, Genexosome entered into and closed an Asset Purchase Agreement with Dr. Zhou, pursuant to which the Company acquired all assets, including all intellectual property and exosome separation systems, held by Dr. Zhou pertaining to the business of researching, developing and commercializing exosome technologies. In consideration of the assets, Genexosome paid Dr. Zhou $876,087 in cash, transferred 500,000 shares of common stock of the Company to Dr. Zhou and issued Dr. Zhou 400 shares of common stock of Genexosome. Further, The Company had not been able to realize the financial projections provided by Dr. Zhou at the time of the acquisition and has decided to impair the intangible asset associated with this acquisition to zero. Dr. Zhou was terminated as Co-CEO of Genexosome on August 14, 2019. Further, on October 28, 2019, Research Institute at Nationwide Children’s Hospital (“Research Institute”) filed a Complaint in the United States District Court for the Southern District of Ohio Eastern Division against Dr. Zhou, Li Chen, the Company and Genexosome with various claims against the Company and Genexosome including misappropriation of trade secrets in violation of the Defend Trade Secrets Act of 2016 and violation of Ohio Uniform Trade Secrets Act. Research Institute is seeking monetary damages, injunctive relief, exemplary damages, injunctive relief and other equitable relief. The Company intends to vigorously defend against this action and pursue all available legal remedies. The criminal proceedings against Dr. Zhou and Li Chen have been concluded. The Company, Genexosome and the Research Institute entered into a settlement agreement dated June 7, 2022 (the “Settlement Agreement”), whereby the Company agreed to pay the Research Institute $450,000 on each of the sixty-day, one year and two-year anniversaries of the Settlement Date. In addition, the Company agreed to pay the Research Institute 30% of the Company’s initial pre-tax profit of $3,333,333, 20% of the Company’s second pre-tax profit of $3,333,333 and 10% of the Company’s third pre-tax profit of $3,333,333. The parties provided a mutual release as well.
ITEM 4. MINE SAFETY DISCLOSURES
None.
50
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock has been listed on the Nasdaq Capital Market under the symbol “ALBT” since November 10, 2022. Our common stock was listed on the Nasdaq Capital Market under the symbol “AVCO” from November 5, 2018 through the close of business on November 9, 2022. Our common shares were traded previously on the OTC Market Group Inc.’s Venture Market (the “OTCQB”) since February 22, 2016, under the symbol “AVCO” since October 18, 2016 and “GTHC” prior to October 18, 2016.
Holders of Record
As of March 30, 2023, there were approximately 222 registered holders of record of our shares of common stock, based upon information received from our stock transfer agent. However, this number does not include beneficial owners whose shares were held of record by nominees or broker dealers.
ITEM 6. [RESERVED]
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations for the years ended December 31, 2022 and 2021 should be read in conjunction with our consolidated financial statements and related notes to those consolidated financial statements that are included elsewhere in this report. Certain information contained in the discussion and analysis set forth below includes forward-looking statements that involve risks and uncertainties.
Special Note Regarding Forward-looking Statements
All statements other than statements of historical fact included in this Form 10-K including, without limitation, statements under “Management’s Discussion and Analysis of Financial Condition and Results of Operations” regarding our financial position, business strategy and the plans and objectives of management for future operations, are forward-looking statements. When used in this Form 10-K, words such as “anticipate,” “believe,” “estimate,” “expect,” “intend” and similar expressions, as they relate to us or our management, identify forward-looking statements. Such forward-looking statements are based on the beliefs of management, as well as assumptions made by, and information currently available to, our management. Actual results could differ materially from those contemplated by the forward-looking statements as a result of a number of factors, including those set forth under the risk factors and business sections in this Form 10-K.
Impact of COVID-19 on Our Operations, Financial Condition, Liquidity and Results of Operations
Although the COVID-19 vaccines have generally been introduced to the public, the ultimate impact of the COVID-19 pandemic on our operations is unknown and will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration of the COVID-19 outbreak, new information which may emerge concerning the severity of the COVID-19 pandemic, a significant increase in new and variant strains of COVID-19 cases, availability and effectiveness of COVID-19 vaccines and therapeutics, the level of acceptance of the vaccine by the general population and any additional preventative and protective actions that governments, or us, may determine are needed.
51
The occurrence of COVID-19 pandemic had negative impact on our operations. Some of the universities and laboratories with which we collaborate were temporarily closed. Our general development operations have continued during the COVID-19 pandemic and we have not had significant disruption. However, we are uncertain if the COVID-19 pandemic will impact future operations at our laboratory, or our ability to collaborate with other laboratories and universities. In addition, we are unsure if the COVID-19 pandemic will impact future clinical trials. Given the dynamic nature of these circumstances, the duration of business disruption and reduced traffic, the related financial effect cannot be reasonably estimated at this time.
We have limited cash available to fund planned operations and although we have other sources of capital described below under “Liquidity and Capital Resources,” management continues to pursue various financing alternatives to fund our operations so we can continue as a going concern. However, the COVID-19 pandemic has created significant economic uncertainty and volatility in the credit and capital markets. Management plans to secure the necessary financing through the issue of new equity and/or the entering into of strategic partnership arrangements but the ultimate impact of the COVID-19 pandemic on our ability to raise additional capital is unknown and will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration of the COVID-19 outbreak and new information which may emerge concerning the severity of the COVID-19 pandemic. We may not be able to raise sufficient additional capital and may tailor our operations based on the amount of funding we are able to raise in the future. Nevertheless, there is no assurance that these initiatives will be successful. Further, there is no assurance that capital available to us in any future financing will be on acceptable terms.
Overview
The Company is a clinical-stage biotechnology company dedicated to developing and delivering innovative, transformative cellular therapeutics, precision diagnostics, and clinical laboratory services. The Company also provides strategic advisory and outsourcing services to facilitate and enhance its clients’ growth and development, as well as competitiveness in healthcare and CellTech industry markets. Through its subsidiary structure with unique integration of verticals from innovative research and development to automated bioproduction and accelerated clinical development, the Company is establishing a leading role in the fields of cellular immunotherapy (including CAR-T/NK), exosome technology (ACTEX™), and regenerative therapeutics.
Avalon achieves and fosters seamless integration of unique verticals to bridge and accelerate innovative research, bio-process development, clinical programs and product commercialization. Avalon’s upstream innovative research includes:
| ● | Development of Avalon Clinical-grade Tissue-specific Exosome (“ACTEX™”); |
| ● | Novel therapeutic and diagnostic targets development utilizing QTY-code protein design technology with Massachusetts Institute of Technology (MIT) including using the QTY code protein design technology for development of a hemofiltration device to treat Cytokine Storm; |
| ● | Co-development of next generation, mRNA-based immune effector cell therapeutic modalities with Arbele Limited. |
Avalon’s midstream bio-processing and bio-production facility is co-developed at the University of Pittsburgh Medical Center (UPMC) with state-of-the-art infrastructure and standardization accredited with cGMP, FACT, aaBB, CLIA and CAP, as well as stringent QC/QA facility for standardized bio-manufacturing of clinical-grade cellular products involved in our clinical programs in immune effector cell therapy and ACTEX-based regenerative therapeutics.
52
Avalon’s downstream medical team and facility consists of top-rated affiliated hospital network and experts specialized in hematology, oncology, cellular immunotherapy, hematopoietic stem/progenitor cell transplant, as well as regenerative therapeutics. Our major clinical programs include:
| ● | AVA-001: Avalon has initiated its first-in-human clinical trial of CD19 CAR-T candidate, AVA-001 in August 2019 at the Hebei Yanda Lu Daopei Hospital and Beijing Lu Daopei Hospital in China (the world’s single largest CAR-T treatment network with over 1,200 patients being treated with CAR-T) for the indication of relapsed/refractory B-cell acute lymphoblastic leukemia and non-Hodgkin Lymphoma). The AVA-001 candidate (co-developed with China Immunotech Co. Ltd) is characterized by the utilization of 4-1BB (CD137) co-stimulatory signaling pathway, conferring a strong anti-cancer activity during pre-clinical study. It also features a shorter bio-manufacturing time which leads to the advantage of prompt treatment to patients where timing is important related hematologic malignancies. Avalon has successfully completed the first-in-human clinical trial of its AVA-001 anti-CD19 CAR-T cell therapy as a bridge to allogeneic bone marrow transplantation for patients with relapsed/refractory B-cell acute lymphoblastic leukemia at the Lu Daopei Hospital (registered clinical trial number NCT03952923) with excellent efficacy (90% complete remission rate) and minimal adverse side effects. Avalon is currently expanding the patient recruitment and indication for AVA-001 to include relapsed/refractory non-Hodgkin lymphoma patients. |
| ● | AVA-011 and FLASH-CAR™: The Company advanced its next generation immune cell therapy using RNA-based, non-viral FLASH-CAR™ technology co-developed with the Company’s strategic partner Arbele Limited. The multiplex FLASH-CAR™ platform can be used to create personalized (“autologous’) cell therapy from a patient’s own cells, as well as “off-the-shelf” cell therapy from a universal donor. Our leading candidate, AVA-011, is a dual-target (anti-CD19/CD22) CAR-T which has completed pre-clinical research stage, and currently at IND-enabling process development stage at UPMC (Dr. Yen-Michael Hsu as Principal Investigator) to generate clinical-grade cell-therapy products for subsequent clinical studies. |
| ● | ACTEX™: Stem cell-derived Avalon Clinical-grade Tissue-specific Exosomes (ACTEX™) is one of the core technology platforms that has been co-developed by Avalon GloboCare and the University of Pittsburgh Medical Center. The Company formed a strategic partnership with HydroPeptide, LLC, a leading epigenetics skin care company, to engage in co-development and commercialization of a series of clinical-grade, exosome-based cosmeceutical and orthopedic products. As part of this agreement, the Company signed a three-way Material Transfer Agreement between Avalon GloboCare, HydroPeptide and the University of Pittsburgh Medical Center. |
| ● | AVA-Trap™: Avalon’s AVA-Trap™ therapeutic program plans to enter animal model testing followed by expedited clinical studies with the goal of providing an effective therapeutic option to combat COVID-19 and other life-threatening conditions involving cytokine storms. The Company initiated a sponsored research and co-development project with Massachusetts Institute of Technology (MIT) led by Professor Shuguang Zhang as Principal Investigator in May 2019. Using the unique QTY code protein design platform, six water-soluble variant cytokine receptors have been successfully designed and tested to show binding affinity to the respective cytokines. |
Going Concern
The Company is a clinical-stage biotechnology company dedicated to developing and delivering innovative, transformative cellular therapeutics, precision diagnostics, and clinical laboratory services. The Company also provides strategic advisory and outsourcing services to facilitate and enhance its clients’ growth and development, as well as competitiveness in healthcare and CellTech industry markets. Through its subsidiary structure with unique integration of verticals from innovative research and development to automated bioproduction and accelerated clinical development, the Company is establishing a leading role in the fields of cellular immunotherapy (including CAR-T/NK), exosome technology (ACTEX™), and regenerative therapeutics.
In addition, the Company owns commercial real estate that houses its headquarters in Freehold, New Jersey. These consolidated financial statements have been prepared assuming that the Company will continue as a going concern, which contemplates, among other things, the realization of assets and the satisfaction of liabilities in the normal course of business.
53
As reflected in the accompanying consolidated financial statements, the Company had working capital deficit of $1,206,279 at December 31, 2022 and had incurred recurring net losses and generated negative cash flow from operating activities of $11,930,847 and $7,037,224 for the year ended December 31, 2022, respectively. The Company has a limited operating history and its continued growth is dependent upon the generating rental revenue from its income-producing real estate property in New Jersey and obtaining additional financing to fund future obligations and pay liabilities arising from normal business operations. In addition, the current cash balance cannot be projected to cover the operating expenses for the next twelve months from the release date of this report. These matters raise substantial doubt about the Company’s ability to continue as a going concern. The ability of the Company to continue as a going concern is dependent on the Company’s ability to raise additional capital, implement its business plan, and generate significant revenues. There are no assurances that the Company will be successful in its efforts to generate significant revenues, maintain sufficient cash balance or report profitable operations or to continue as a going concern. The Company plans on raising capital through the sale of equity to implement its business plan. However, there is no assurance these plans will be realized and that any additional financings will be available to the Company on satisfactory terms and conditions, if any.
The occurrence of an uncontrollable event such as the COVID-19 pandemic had negatively impact on the Company’s operations. Our general development operations have continued during the COVID-19 pandemic and we have not had significant disruption. However, we are uncertain if the COVID-19 pandemic will impact future operations at our laboratory, or our ability to collaborate with other laboratories and universities. In addition, we are unsure if the COVID-19 pandemic will impact future clinical trials. Given the dynamic nature of these circumstances, the duration of business disruption and reduced traffic, the related financial effect cannot be reasonably estimated at this time.
The accompanying consolidated financial statements do not include any adjustments related to the recoverability or classification of asset-carrying amounts or the amounts and classification of liabilities that may result should the Company be unable to continue as a going concern.
Critical Accounting Policies
Use of Estimates
The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Changes in these estimates and assumptions may have a material impact on the consolidated financial statements and accompanying notes. Making estimates requires management to exercise significant judgment. It is at least reasonably possible that the estimate of the effect of a condition, situation or set of circumstances that existed at the date of the financial statements, which management considered in formulating its estimate, could change in the near term due to one or more future confirming events. Accordingly, the actual results could differ significantly from those estimates. Significant estimates during the years ended December 31, 2022 and 2021 include the useful life of property and equipment and investment in real estate, assumptions used in assessing impairment of long-term assets, valuation of deferred tax assets and the associated valuation allowances, valuation of stock-based compensation, and assumptions used to determine fair value of warrants and embedded conversion features of convertible note payable.
Revenue Recognition
The Company recognizes revenue under Accounting Standards Codification (“ASC”) Topic 606, Revenue from Contracts with Customers (“ASC 606”). The core principle of the revenue standard is that a company should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. The following five steps are applied to achieve that core principle:
| ● | Step 1: Identify the contract with the customer |
| ● | Step 2: Identify the performance obligations in the contract |
54
| ● | Step 3: Determine the transaction price |
| ● | Step 4: Allocate the transaction price to the performance obligations in the contract |
| ● | Step 5: Recognize revenue when the company satisfies a performance obligation |
In order to identify the performance obligations in a contract with a customer, a company must assess the promised goods or services in the contract and identify each promised goods or service that is distinct. A performance obligation meets ASC 606’s definition of a “distinct” goods or service (or bundle of goods or services) if both of the following criteria are met:
| ● | The customer can benefit from the goods or service either on its own or together with other resources that are readily available to the customer (i.e., the goods or service is capable of being distinct). |
| ● | The entity’s promise to transfer the goods or service to the customer is separately identifiable from other promises in the contract (i.e., the promise to transfer the goods or service is distinct within the context of the contract). |
If a goods or service is not distinct, the goods or service is combined with other promised goods or services until a bundle of goods or services is identified that is distinct.
The transaction price is the amount of consideration to which an entity expects to be entitled in exchange for transferring promised goods or services to a customer, excluding amounts collected on behalf of third parties (for example, some sales taxes). The consideration promised in a contract with a customer may include fixed amounts, variable amounts, or both. Variable consideration is included in the transaction price only to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved.
The transaction price is allocated to each performance obligation on a relative standalone selling price basis. The transaction price allocated to each performance obligation is recognized when that performance obligation is satisfied, at a point in time or over time as appropriate.
The Company’s revenues are derived from providing medial related consulting services for its’ related parties. Revenues related to its service offerings are recognized at a point in time when service is rendered. Any payments received in advance of the performance of services are recorded as deferred revenue until such time as the services are performed.
The Company has determined that the ASC 606 does not apply to rental contracts, which are within the scope of other revenue recognition accounting standards.
Rental income from operating leases is recognized on a straight-line basis under the guidance of ASC 842. Lease payments under tenant leases are recognized on a straight-line basis over the term of the related leases. The cumulative difference between lease revenue recognized under the straight-line method and contractual lease payments are included in rent receivable on the consolidated balance sheets.
The Company does not offer promotional payments, customer coupons, rebates or other cash redemption offers to its customers.
Income Taxes
We are governed by the income tax laws of China and the United States. Income taxes are accounted for pursuant to ASC 740 “Accounting for Income Taxes,” which is an asset and liability approach that requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in our financial statements or tax returns. The charge for taxes is based on the results for the period as adjusted for items, which are non-assessable or disallowed. It is calculated using tax rates that have been enacted or substantively enacted by the balance sheet date.
Deferred tax is accounted for using the balance sheet liability method in respect of temporary differences arising from differences between the carrying amount of assets and liabilities in the financial statements and the corresponding tax basis used in the computation of assessable tax profit. In principle, deferred tax liabilities are recognized for all taxable temporary differences, and deferred tax assets are recognized to the extent that it is probably that taxable profit will be available against which deductible temporary differences can be utilized.
Deferred tax is calculated using tax rates that are expected to apply to the period when the asset is realized or the liability is settled. Deferred tax is charged or credited in the income statement, except when it is related to items credited or charged directly to equity, in which case the deferred tax is changed to equity. Deferred tax assets and liabilities are offset when they related to income taxes levied by the same taxation authority and we intend to settle its current tax assets and liabilities on a net basis.
55
Recent Accounting Standards
For details of applicable new accounting standards, please, refer to Recent Accounting Standards in Note 3 of our consolidated financial statements accompanying this report.
RESULTS OF OPERATIONS
Comparison of Results of Operations for the Years Ended December 31, 2022 and 2021
Revenues
For the year ended December 31, 2022, we had real property rental revenue of $1,202,169, as compared to $1,203,560 for the year ended December 31, 2021, a decrease of $1,391, or 0.1%. We expect that our revenue from real property rent will remain in its current level with minimal increase in the near future.
For the year ended December 31, 2022, we did not have any medical related consulting services revenue since there was no demand for our consulting service from our related parties and there were no orders for our medical related consulting services from third party in 2022. Due to the winding down of the medical related consulting services segment in 2022, the Company decided to cease all operations of this segment and no longer has any material revenues or expenses in this segment. For the year ended December 31, 2021, we had medical related consulting services revenue from related party of $187,412.
Costs and Expenses
Real property operating expenses consist of property management fees, property insurance, real estate taxes, depreciation, repairs and maintenance fees, utilities and other expenses related to our rental properties.
For the year ended December 31, 2022, our real property operating expenses amounted to $929,441, as compared to $829,287 for the year ended December 31, 2021, an increase of $100,154, or 12.1%. The increase was mainly due to an increase in building cleaning fees of approximately $15,000, an increase in property management fees of approximately $21,000, an increase in repairs and maintenance fee of approximately $32,000, an increase in utilities of approximately $30,000, and an increase in other miscellaneous items of approximately $2,000.
Costs of medical related consulting services include the cost of labor and related benefits, travel expenses related to medical related consulting services, and other overhead costs.
There were no comparative revenue and related costs of revenue from our medical related consulting services for the year ended December 31, 2022 since there was no demand for our consulting service from our related parties and there were no orders for our medical related consulting services from third party in 2022. For the year ended December 31, 2021, costs of medical related consulting services amounted to $147,167.
Real Property Operating Income
Our real property operating income for the year ended December 31, 2022 was $272,728, representing a decrease of $101,545, or 27.1%, as compared to $374,273 for the year ended December 31, 2021. The decrease was primarily attributable to the increase in real property operating expenses as described above. We expect our real property operating income will remain in its current level with minimal increase in the near future.
Gross Profit from Medical Related Consulting Services and Gross Margin
We did not generate any gross profit from medical related consulting services in the year ended December 31, 2022. Our gross profit from medical related consulting services for the year ended December 31, 2021 was $40,245, with a gross margin of 21.5%.
56
Other Operating Expenses
For the years ended December 31, 2022 and 2021, other operating expenses consisted of the following:
| Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Advertising and marketing | $ | 1,325,313 | $ | 328,565 | ||||
| Professional fees | 2,909,652 | 4,946,696 | ||||||
| Compensation and related benefits | 1,863,188 | 2,042,278 | ||||||
| Research and development | 731,328 | 1,025,009 | ||||||
| Litigation settlement | 1,350,000 | - | ||||||
| Directors and officers liability insurance premium | 414,757 | 367,365 | ||||||
| Travel and entertainment | 163,213 | 156,483 | ||||||
| Rent and related utilities | 77,352 | 78,547 | ||||||
| Other general and administrative | 230,820 | 303,405 | ||||||
| $ | 9,065,623 | $ | 9,248,348 | |||||
| ● | For the year ended December 31, 2022, advertising and marketing expenses increased by $996,748 or 303.4% as compared to the year ended December 31, 2021. The increase was primarily due to increased advertising activities to enhance the visibility and marketability of our company and to improve brand recognition and awareness. We expect that our advertising expenses will remain in its current level with minimal increase in the near future. |
| ● | Professional fees primarily consisted of accounting fees, audit fees, legal service fees, consulting fees, investor relations service charges, valuation service fees and other fees. For the year ended December 31, 2022, professional fees decreased by $2,037,044, or 41.2%, as compared to the year ended December 31, 2021, which was primarily attributable to a decrease in consulting fees of approximately $1,648,000 mainly due to the decrease in use of consulting service providers, a decrease in legal service fees of approximately $262,000 mainly due to the decrease in use of legal service providers related to the acquisition of a British Virgin Island company which was terminated on January 1, 2022, and a decrease in one time valuation service fees of $180,000, offset by an increase in other miscellaneous items of approximately $53,000. We expect that our professional fees will remain in its current level with minimal increase in the near future. |
| ● | For the year ended December 31, 2022, compensation and related benefits decreased by $179,090, or 8.8%, as compared to the year ended December 31, 2021, which was primarily attributable to the decrease in stock-based compensation which reflected the value of options granted and vested to our management. We expect that our compensation and related benefits will remain in its current level with minimal increase in the near future. |
| ● | For the year ended December 31, 2022, research and development expenses decreased by $293,681, or 28.7%, as compared to the year ended December 31, 2021. The decrease was mainly attributable to decreased research and development projects in year 2022. We expect that our research and development expenses will remain in its current level with minimal decrease in the near future. |
| ● | For the year ended December 31, 2022, litigation settlement increased by $1,350,000, or 100.0%, as compared to the year ended December 31, 2021. The increase was due to a settlement signed in June 2022 related to Research Institute litigation. |
| ● | For the year ended December 31, 2022, Directors and Officers Liability Insurance premium increased by $47,392, or 12.9%, as compared to the year ended December 31, 2021. The increase was mainly due to different insurance provider with different premium. |
57
| ● | For the year ended December 31, 2022, travel and entertainment expense increased by $6,730, or 4.3%, as compared to the year ended December 31, 2021. The increase was mainly due to increased business travel activities in year 2022. |
| ● | For the year ended December 31, 2022, rent and related utilities expenses decreased by $1,195, or 1.5%, as compared to the year ended December 31, 2021. |
| ● | Other general and administrative expenses mainly consisted of NASDAQ listing fee, office supplies, and other miscellaneous items. For the year ended December 31, 2022, other general and administrative expenses decreased by $72,585, or 23.9%, as compared to the year ended December 31, 2021. The decrease was mainly attributable to a decrease in depreciation of approximately $19,000, which was primarily due to certain office equipment and furniture had reached the end of depreciation period and no further depreciation is required for these fixed assets in year 2022, a decrease in office supplies of approximately $13,000, and a decrease in other miscellaneous items of approximately $40,000 due to our efforts at stricter controls on corporate expenditure. |
Loss from Operations
As a result of the foregoing, for the year ended December 31, 2022, loss from operations amounted to $8,792,895, as compared to $8,833,830 for the year ended December 31, 2021, a decrease of $40,935 or 0.5%.
Other (Expense) Income
Other (expense) income mainly includes third party and related party interest expense, conversion inducement expense, loss from equity method investment, change in fair value of derivative liability, and other miscellaneous income.
Other expense, net, totaled $3,137,952 for the year ended December 31, 2022, as compared to $256,669 for the year ended December 31, 2021, an increase of $2,881,283, or 1,122.6%, which was primarily attributable to an increase in third party interest expense of approximately $3,496,000 mainly driven by the amortization of debt discount and debt issuance cost of approximately $3,311,000 and the increased interest expense of approximately $186,000 from third party debts in year 2022, and an increase in conversion inducement expense of approximately $344,000 resulted from the reduction in the conversion price, offset by an increase in gain from change in fair value of derivative liability of approximately $601,000, an increase in other miscellaneous income of approximately $219,000, mainly driven by reagent sale in year 2022, a decrease in interest expense – related party of approximately $121,000 due to the decrease in outstanding borrowing in year 2022, and a decrease in loss from equity method investment of approximately $19,000.
Income Taxes
We did not have any income taxes expense for the years ended December 31, 2022 and 2021 since we incurred losses in these periods.
Net Loss
As a result of the factors described above, our net loss was $11,930,847 for the year ended December 31, 2022, as compared to $9,090,499 for the year ended December 31, 2021, an increase of $2,840,348 or 31.2%.
58
Net Loss Attributable to Avalon GloboCare Corp. Common Shareholders
The net loss attributable to Avalon GloboCare Corp. common shareholders was $11,930,847 or $1.28 per share (basic and diluted) for the year ended December 31, 2022, as compared with $9,090,499 or $1.07 per share (basic and diluted) for the year ended December 31, 2021, an increase of $2,840,348 or 31.2%.
Foreign Currency Translation Adjustment
Our reporting currency is the U.S. dollar. The functional currency of our parent company, AHS, Avalon RT 9, Genexosome, Avactis, and Exosome, is the U.S. dollar and the functional currency of Avalon Shanghai is the Chinese Renminbi (“RMB”). The financial statement of our subsidiary whose functional currency is the RMB are translated to U.S. dollars using period end rate of exchange for assets and liabilities, average rate of exchange for revenues, costs, and expenses and cash flows, and at historical exchange rate for equity. Net gains and losses resulting from foreign exchange transactions are included in the results of operations. As a result of foreign currency translations, which are a non-cash adjustment, we reported a foreign currency translation loss of $47,871 and a foreign currency translation gain of $ 25,244 for the years ended December 31, 2022 and 2021, respectively. This non-cash loss/gain had the effect of increasing/decreasing our reported comprehensive loss.
Comprehensive Loss
As a result of our foreign currency translation adjustment, we had comprehensive loss of $11,978,718 and $9,065,255 for the years ended December 31, 2022 and 2021, respectively.
Liquidity and Capital Resources
The Company has a limited operating history and its continued growth is dependent upon generating rental revenue from its income-producing real estate property in New Jersey and obtaining additional financing to fund future obligations and pay liabilities arising from normal business operations. In addition, the current cash balance cannot be projected to cover the operating expenses for the next twelve months from the release date of this report. These matters raise substantial doubt about the Company’s ability to continue as a going concern. The ability of the Company to continue as a going concern is dependent on the Company’s ability to raise additional capital, implement its business plan, and generate significant revenues. There are no assurances that the Company will be successful in its efforts to generate significant revenues, maintain sufficient cash balance or report profitable operations or to continue as a going concern. The Company plans on raising capital through the sale of equity to implement its business plan. However, there is no assurance these plans will be realized and that any additional financings will be available to the Company on satisfactory terms and conditions, if any.
The occurrence of an uncontrollable event such as the COVID-19 pandemic is likely to negatively affect the Company’s operations. Efforts to contain the spread of the coronavirus have intensified, including social distancing, travel bans and quarantine, and these are likely to negatively impact our tenants, employees and consultants. These, in turn, will not only impact our operations, financial condition and demand for our medical related consulting services but our overall ability to react timely to mitigate the impact of this event. Given the dynamic nature of these circumstances, the duration of business disruption and reduced traffic, the related financial effect cannot be reasonably estimated at this time.
59
Liquidity is the ability of a company to generate funds to support its current and future operations, satisfy its obligations and otherwise operate on an ongoing basis. At December 31, 2022 and 2021, we had cash balance of approximately $1,991,000 and $808,000, respectively. These funds are kept in financial institutions located as follows:
| Country: | December 31, 2022 | December 31, 2021 | ||||||||||||||
| United States | $ | 1,806,083 | 90.7 | % | $ | 767,605 | 95.1 | % | ||||||||
| China | 184,827 | 9.3 | % | 39,933 | 4.9 | % | ||||||||||
| Total cash | $ | 1,990,910 | 100.0 | % | $ | 807,538 | 100.0 | % | ||||||||
Under applicable PRC regulations, foreign invested enterprises, or FIEs, in China may pay dividends only out of their accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. In addition, a foreign invested enterprise in China is required to set aside at least 10% of its after-tax profit based on PRC accounting standards each year to its general reserves until the cumulative amount of such reserves reach 50% of its registered capital. These reserves are not distributable as cash dividends.
In addition, a small portion of our assets are denominated in RMB, which is not freely convertible into foreign currencies. All foreign exchange transactions take place either through the People’s Bank of China or other banks authorized to buy and sell foreign currencies at the exchange rates quoted by the People’s Bank of China. Approval of foreign currency payments by the People’s Bank of China or other regulatory institutions requires submitting a payment application form together with suppliers’ invoices, shipping documents and signed contracts. These currency exchange control procedures imposed by the PRC government authorities may restrict the ability of our PRC subsidiary to transfer its net assets to the Parent Company through loans, advances or cash dividends.
The current PRC Enterprise Income Tax (“EIT”) Law and its implementing rules generally provide that a 10% withholding tax applies to China-sourced income derived by non-resident enterprises for PRC enterprise income tax purposes unless the jurisdiction of incorporation of such enterprises’ shareholder has a tax treaty with China that provides for a different withholding arrangement.
The following table sets forth a summary of changes in our working capital deficit from December 31, 2021 to December 31, 2022:
| December 31, | Changes in | |||||||||||||||
| 2022 | 2021 | Amount | Percentage | |||||||||||||
| Working capital deficit: | ||||||||||||||||
| Total current assets | $ | 2,373,526 | $ | 1,323,042 | $ | 1,050,484 | 79.4 | % | ||||||||
| Total current liabilities | 3,579,805 | 4,401,658 | (821,853 | ) | (18.7 | )% | ||||||||||
| Working capital deficit | $ | (1,206,279 | ) | $ | (3,078,616 | ) | $ | 1,872,337 | (60.8 | )% | ||||||
Our working capital deficit decreased by $1,872,337 to $1,206,279 at December 31, 2022 from $3,078,616 at December 31, 2021. The decrease in working capital deficit was primarily attributable to an increase in cash of approximately $1,183,000 mainly due to the issuance of convertible debt and balloon promissory note in year 2022, a decrease in accrued professional fees of approximately $208,000 which was mainly due to payments made to our professional service providers in the year ended December 31, 2022, a decrease in accrued research and development fees of approximately $90,000 resulting from payments made to research and development service providers in the year ended December 31, 2022, a decrease in accrued payroll liability and directors’ compensation of approximately $83,000, a decrease in accrued liabilities and other payables – related parties of approximately $368,000 which was mainly attributable to the accrued and unpaid related party interest was settled in shares in the year ended December 31, 2022, a decrease in operating lease obligation of approximately $140,000, a decrease in note payable – related party of $390,000 due to repayment made to this related party in the year ended December 31, 2022, offset by a decrease in other current assets of approximately $200,000, which was mainly attributable to the decrease in prepaid professional fee of approximately $93,000, which were recognized as expense over the related service period in year 2022, and the decrease in recoverable VAT of approximately $20,000 and the decrease in other miscellaneous items of approximately $87,000, and an increase in accrued settlement of lawsuit of $450,000 due to a settlement signed in June 2022.
60
Because the exchange rate conversion is different for the consolidated balance sheets and the consolidated statements of cash flows, the changes in assets and liabilities reflected on the consolidated statements of cash flows are not necessarily identical with the comparable changes reflected on the consolidated balance sheets.
Cash Flows for the Year Ended December 31, 2022 Compared to the Year Ended December 31, 2021
The following summarizes the key components of our cash flows for the years ended December 31, 2022 and 2021:
| Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Net cash used in operating activities | $ | (7,037,224 | ) | $ | (5,024,479 | ) | ||
| Net cash used in investing activities | (9,053,470 | ) | (68,135 | ) | ||||
| Net cash provided by financing activities | 17,263,989 | 5,170,132 | ||||||
| Effect of exchange rate on cash and restricted cash | 10,077 | 3,443 | ||||||
| Net increase in cash and restricted cash | $ | 1,183,372 | $ | 80,961 | ||||
Net cash flow used in operating activities for the year ended December 31, 2022 was $7,037,224, which primarily reflected our consolidated net loss of approximately $11,931,000, and the non-cash item adjustment consisting of change in fair market value of derivative liability of approximately $601,000, and the changes in operating assets and liabilities, primarily consisting of a decrease in operating lease obligation of approximately $142,000, offset by an increase in accrued liabilities and other payables of approximately $331,000, an increase in accrued liabilities and other payables – related parties of approximately $80,000, and the non-cash items adjustment primarily consisting of depreciation of approximately $331,000, amortization of right-of-use asset of approximately $136,000, stock-based compensation and service expense of approximately $1,107,000, amortization of debt discount of approximately $3,281,000 mainly resulting from the conversion of convertible debt in July 2022, and conversion inducement expense of approximately $344,000 resulted from the reduction in the conversion price.
Net cash flow used in operating activities for the year ended December 31, 2021 was $5,024,479, which primarily reflected our consolidated net loss of approximately $9,090,000, and the changes in operating assets and liabilities, primarily consisting of a decrease in operating lease obligation of approximately $121,000, offset by an increase accrued liabilities and other payables of approximately $1,331,000, which was mainly attributable the increase in accrued professional fees of approximately $669,000 due to increased professional service providers, the increase in accrued research and development fees of approximately $415,000 which was primarily attributable to we increased research and development projects in 2021, and the increase in accrued payroll liability and directors’ compensation of approximately $153,000, and an increase in accrued liabilities and other payables – related parties of approximately $200,000 resulting from the increase in accrued interest for related party borrowings, and the non-cash items adjustment primarily consisting of depreciation of approximately $312,000, amortization of right-of-use asset of approximately $127,000, and stock-based compensation and service expense of approximately $2,110,000.
61
We expect our cash used in operating activities to increase due to the following:
| ● | the development and commercialization of new products; |
| ● | an increase in professional staff and services; and |
| ● | an increase in public relations and/or sales promotions for existing and/or new brands as we expand within existing markets or enter new markets. |
Net cash flow used in investing activities was $5,053,748 for the year ended December 31, 2022 as compared to $68,135 for the year ended December 31, 2021. During the year ended December 31, 2022, we made payments for purchase of property and equipment of approximately $2,000 and made additional investment in Epicon equity method investment of approximately $52,000 and made payments for acquisition of 40% interest in Laboratory Services MSO, LLC of approximately $9,000,000. During the year ended December 31, 2021, we made payments for purchase of property and equipment of approximately $18,000 and for improvement of commercial real estate of approximately $10,000, and made additional investment in equity method investment of approximately $40,000.
Net cash flow provided by financing activities was $17,263,989 for the year ended December 31, 2022 as compared to $5,170,132 for the year ended December 31, 2021. During the year ended December 31, 2022, we received proceeds from related party borrowings of $100,000, and proceeds from issuance of convertible debt and warrants of approximately $3,719,000, and net proceeds from issuance of balloon promissory note of approximately $4,534,000 (net of cash paid for debt issuance costs of approximately $266,000), and net proceeds from equity offering of approximately $712,000 (net of cash paid for commission and other offering costs of approximately $24,000), and proceeds from issuance of Series A preferred stock of $9,000,000 to fund our working capital needs, offset by repayments made for note payable – related party of $390,000 and repayments made for loan payable – related party of $410,000. During the year ended December 31, 2021, we received proceeds from related party borrowings of approximately $2,550,000 and net proceeds from equity offering of approximately $2,620,000 (net of cash paid for commission and other offering costs of approximately $240,000) to fund our working capital needs.
Our capital requirements for the next twelve months primarily relate to working capital requirements, including salaries, fees related to third parties’ professional services, reduction of accrued liabilities, mergers, acquisitions and the development of business opportunities. These uses of cash will depend on numerous factors including our revenues and our ability to control costs. All funds received have been expended in the furtherance of growing the business. The following trends are reasonably likely to result in a material decrease in our liquidity over the near to long term:
| ● | an increase in working capital requirements to finance our current business, including ongoing research and development programs, clinical studies, as well as commercial strategies; |
| ● | the use of capital for mergers, acquisitions and the development of business opportunities; |
| ● | addition of administrative personnel as the business grows; and |
| ● | the cost of being a public company. |
In the third quarter of 2019, we had secured a $20 million credit facility (Line of Credit) provided by our Chairman, Wenzhao Lu. The unsecured credit facility bears interest at a rate of 5% and provides for maturity on drawn loans 36 months after funding. As of December 31, 2022, the total principal amount outstanding under the Credit Line was $0 and we used approximately $5.9 million of the credit facility and have approximately $14.1 million remaining available under the Line Credit.
62
On December 13, 2019, we entered into an Open Market Sale AgreementSM (the “Sales Agreement”) with Jefferies LLC, as sales agent (“Jefferies”), pursuant to which we may offer and sell, from time to time, through Jefferies, shares of our common stock, par value $0.0001 per share, having an aggregate offering price of up to $20.0 million. On April 6, 2020, the date on which we filed our Annual Report on Form 10-K for the fiscal year ended December 31, 2019, our registration statement became subject to the offering limits set forth in General Instruction I.B.6 of Form S-3. As of April 6, 2020, the aggregate market value of our outstanding common stock held by non-affiliates, or public float, was $39,564,237, based on 2,369,116 shares of our outstanding common stock that were held by non-affiliates on such date and a price of $16.7 per share, which was the price at which our common stock was last sold on The Nasdaq Capital Market on February 19, 2020 (a date within 60 days of the date hereof), calculated in accordance with General Instruction I.B.6 of Form S-3. We have not offered any securities pursuant to General Instruction I.B.6 of Form S-3 in the 12 calendar months preceding the date of this prospectus supplement. We filed a prospectus supplement to amend and supplement the information in our prospectus and original prospectus supplement based on the amount of securities that we are eligible to sell under General Instruction I.B.6 of Form S-3. After giving effect to the $13,000,000 offering limit imposed by General Instruction I.B.6 of Form S-3, we may offer and sell additional shares of our common stock having an aggregate offering price of up to $13,000,000 from time to time through Jefferies acting as our sales agent in accordance with the terms of the sales agreement. As of December 31, 2022, we sold a total of 642,949 shares of our common stock through Jefferies with an aggregate offering price of $10,073,707 and we have approximately $4.9 million offering price remaining available under the Sales Agreement.
We estimate that based on current plans and assumptions, that our available cash will be insufficient to satisfy our cash requirements under our present operating expectations through cash available under our Credit Line and sales of equity through our Sales Agreement. Under the Line of Credit, the Company received a loan from the Lender of $750,000 in March 2023. Other than funds received from the sale of our equity and advances from our related party, and cash resource generating from our operations, we presently have no other significant alternative source of working capital. We have used these funds to fund our operating expenses, pay our obligations and grow our company. We will need to raise significant additional capital to fund our operations and to provide working capital for our ongoing operations and obligations. Therefore, our future operation is dependent on our ability to secure additional financing. Financing transactions may include the issuance of equity or debt securities, obtaining credit facilities, or other financing mechanisms. However, the trading price of our common stock and a downturn in the U.S. equity and debt markets could make it more difficult to obtain financing through the issuance of equity or debt securities. Even if we are able to raise the funds required, it is possible that we could incur unexpected costs and expenses or experience unexpected cash requirements that would force us to seek alternative financing. Furthermore, if we issue additional equity or debt securities, stockholders may experience additional dilution or the new equity securities may have rights, preferences or privileges senior to those of existing holders of our common stock. The inability to obtain additional capital may restrict our ability to grow and may reduce our ability to continue to conduct business operations. If we are unable to obtain additional financing, we will be required to cease our operations. To date, we have not considered this alternative, nor do we view it as a likely occurrence.
63
Off-balance Sheet Arrangements
We presently do not have off-balance sheet arrangements.
Foreign Currency Exchange Rate Risk
In November of 2022, we decided to cease all operations in China with the exception of a small administrative office, Avalon Shanghai. We do not expect nor do we plan that there will be further revenue generated from PRC operations in the foreseeable future. Thus, exchange rate fluctuations between RMB and US dollars do not have a material effect on us. For the years ended December 31, 2022 and 2021, we had an unrealized foreign currency translation loss of approximately $48,000 and an unrealized foreign currency translation gain of approximately $25,000, respectively, because of changes in the exchange rate.
Inflation
The effect of inflation on our revenue and operating results was not significant.
64
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
As a smaller reporting company, as defined in Rule 12b-2 of the Exchange Act, we are not required to provide the information required by this Item.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The financial statements begin on page F-1.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that material information required to be disclosed in our periodic reports filed under the Securities Exchange Act of 1934, as amended, or 1934 Act, is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms and to ensure that such information is accumulated and communicated to our management, including our Chief Executive Officer (“CEO”) and Chief Financial Officer (“CFO”) as appropriate, to allow timely decisions regarding required disclosure. We carried out an evaluation, under the supervision and with the participation of our management, including the principal executive officer and the principal financial officer (principal financial officer), of the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Rule 13(a)-15(e) under the 1934 Act, as of the end of the period covered by this report. Our management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives, and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. During evaluation of disclosure controls and procedures as of December 31, 2022 conducted as part of our annual audit and preparation of our annual financial statements, our management, including our CEO and CFO, conducted an evaluation of the effectiveness of the design and operations of our disclosure controls and procedures and concluded that our disclosure controls and procedures were not effective due to the reasons set forth below.
Management’s Report on Internal Control over Financial Reporting
Management is responsible for the preparation and fair presentation of the financial statements included in this annual report. The financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America and reflect management’s judgment and estimates concerning effects of events and transactions that are accounted for or disclosed.
Management is also responsible for establishing and maintaining adequate internal control over financial reporting. Our internal control over financial reporting includes those policies and procedures that pertain to our ability to record, process, summarize and report reliable data. Management recognizes that there are inherent limitations in the effectiveness of any internal control over financial reporting, including the possibility of human error and the circumvention or overriding of internal control. Accordingly, even effective internal control over financial reporting can provide only reasonable assurance with respect to financial statement presentation. Further, because of changes in conditions, the effectiveness of internal control over financial reporting may vary over time.
65
Management regularly assesses controls and did so most recently for our financial reporting as of December 31, 2022. This assessment was based on criteria for effective internal control over financial reporting described in the Internal Control Integrated Framework issued by the Committee of Sponsoring Organizations (COSO) of the Treadway Commission. Based on this assessment, management has concluded that our internal control over financial reporting was not effective as of December 31, 2022, due to the lack of segregation of duties resulting from our small size and testing of the operating effectiveness of the controls. As a result of our Lab Services transaction in February 2023, we intend to retain additional accounting staff and support to enhance our controls and procedures and, in February 2023, we retained a third party with relevant expertise to support us and assist us in enhancing our internal controls and procedures.
In light of the material weaknesses described above, we performed additional analyses and procedures in order to conclude that our consolidated financial statements for the year ended December 31, 2022 included in this Annual Report on Form 10-K were fairly stated in accordance with US GAAP. Accordingly, management believes that despite our material weakness, our consolidated financial statements for the year ended December 31, 2022 are fairly stated, in all material respects, in accordance with US GAAP.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting, as such term is defined in Rules 13a-15(f) under the Exchange Act, during the quarter ended December 31, 2022 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Attestation Report of the Registered Public Accounting Firm
This Annual Report on Form 10-K does not include an attestation report by our independent registered public accounting firm, regarding internal control over financial reporting. As a smaller reporting company, our internal control over financial reporting was not subject to audit by our independent registered public accounting firm pursuant to rules of the Securities and Exchange Commission that permit us to provide only management’s report.
ITEM 9B. OTHER INFORMATION
None.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS.
Not applicable.
66
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Directors and Executive Officers
Below are the names of and certain information regarding our executive officers and directors as of the date hereof:
| Name | Age | Position | ||
| Wenzhao Lu | 65 | Chairman of the Board of Directors | ||
| David Jin, MD, PhD | 55 | Chief Executive Officer, President and Director | ||
| Meng Li | 45 | Chief Operating Officer and Secretary | ||
| Luisa Ingargiola | 55 | Chief Financial Officer | ||
| Steven A. Sanders | 77 | Director | ||
| Lourdes Felix | 55 | Director | ||
| Wilbert J. Tauzin II | 79 | Director | ||
| William B. Stilley, III | 55 | Director | ||
| Tevi Troy | 55 | Director |
Officers are elected annually by the Board of Directors (subject to the terms of any employment agreement), at our annual meeting, to hold such officer until an officer’s successor has been duly appointed and qualified, unless an officer sooner dies, resigns or is removed by the Board.
The principal occupation and business experience during at least the past five years for our executive officers and directors is as follows:
Wenzhao Lu, Chairman of the Board of Directors
Mr. Wenzhao Lu has served as our Chairman of the Board since October 10, 2016. He is a seasoned healthcare entrepreneur with extensive operational knowledge and experience in US & Asia. He has served as Chairman of the Board for the Daopei Medical Group, or DPMG, since 2010 to December, 2021. Under his leadership, DPMG is operating three top-ranked private hospitals (located in Beijing and Hebei), specialty hematology laboratories, as well as a hematology research institute, with more than 100 partnering and collaborating hospitals in China. DPMG was founded by Professor Daopei Lu, a renowned hematologist pioneering in hematopoietic stem cell transplant and member of the Academy of Engineering in China. Mr. Wenzhao Lu received a Bachelor of Arts from Temple University Tyler School of Arts in 1988 and subsequently worked as senior Art Director at Ogilvy & Mather Advertising Company. Prior to joining DPMG, Mr. Lu served as Chief Operating Officer for BioTime Asia Limited, which is a subsidiary of BioTime, Inc. (NYSE American: BTX) in 2009. Mr. Lu is qualified to serve as a director because of his extensive operational knowledge of, and executive level management experience in, the healthcare industry.
67
David Jin, Chief Executive Officer, President and Director
Dr. David Jin, MD, PhD, has served as our Chief Executive Officer, President and a member of the Board of Directors since September 14, 2016. From 2009 to 2017, Dr. Jin has served as the Chief Medical Officer of BioTime, Inc. (NYSE American: BTX), a clinical stage regenerative medicine company with a focus on pluripotent stem cell technology. Dr. Jin also acts as a senior translational clinician-scientist at the Howard Hughes Medical Institute and the Ansary Stem Cell Center at Weill Cornell Medical College of Cornell University. Prior to his current endeavors, Dr. Jin was Chief Consultant/Advisor for various biotech/pharmaceutical companies regarding hematology, oncology, immunotherapy and stem cell-based technology development. Dr. Jin has been Principle Investigator in more than 15 pre-clinical and clinical trials, as well as author/co-author of over 80 peer-reviewed scientific abstracts, articles, reviews, and book chapters. Dr. Jin studied medicine at SUNY Downstate College of Medicine in Brooklyn, New York. He received his clinical training and subsequent faculty tenure at the New York-Presbyterian Hospital (the teaching hospital for both Cornell and Columbia Universities) in the areas of internal medicine, hematology, and clinical oncology. Dr. Jin was honored as Top Chief Medical Officer by ExecRank in 2012, as well as recognized by Leading Physicians of the World in 2015. Dr. Jin is qualified to serve as a director because of his role with us, and his extensive operational knowledge of, and executive level management experience in, the healthcare industry.
Meng Li, Chief Operating Officer and Secretary
Ms. Meng Li has served as our Chief Operating Officer and Secretary since October 10, 2016 and served as a member of the Board of Directors from October 10, 2016 to July 9, 2018 and from April 5, 2019 through December 30, 2022. Ms. Li has over 15 years of executive experience in international marketing, branding, communications, and media investment consultancy. Ms. Li served as Managing Director at Maxus/GroupM (a WPP Group company) where she was responsible for business P&L and corporate management from 2006 to 2015. Prior to joining Maxus/Group M, Ms. Li worked for Zenith Media (a Publicis Group company) from 2000 to 2006 as Senior Manager. Ms. Li received a Bachelor of Arts in International Economic Law from Dalian Maritime University in China.
Luisa Ingargiola, Chief Financial Officer
Luisa Ingargiola has served as our Chief Financial Officer since February 21, 2017. Ms Ingargiola has significant experience serving as Chief Financial Officer or Audit Chair for multiple NASDAQ and NYSE companies. She currently serves as Director and Audit Chair for several public companies including ElectraMeccanica (NASDAQ:SOLO), Dragonfly Energy (DFLI) andVision Marine (VMAR). From 2007 through 2016, Ms. Ingargiola served as the Chief Financial Officer and then Director at MagneGas Corporation (Nasdaq: MNGA. Prior to 2007, Ms. Ingargiola held various roles as Budget Director and Investment Analyst in several private companies. Ms. Ingargiola graduated in 1989 from Boston University with a Bachelor’s degree in Business Administration and a concentration in Finance. In 1996, she received her MBA in Health Administration from the University of South Florida. Ms. Ingargiola is qualified to serve as a Chief Financial Officer because of her extensive knowledge corporate governance, regulatory requirements, executive leadership and knowledge of, and experience in, financing and M&A transactions.
Steven A. Sanders, Director
Steven A. Sanders has served as a member of the Board of Directors since July 30, 2018. Since January 2017, Mr. Sanders has been Of Counsel to the law firm of Ortoli Rosenstadt LLP. From July 2007 until January 2017, Mr. Sanders was a Senior Partner of Ortoli Rosenstadt LLP. From January 1, 2004 until June 30, 2007, he was Of Counsel to the law firm of Rubin, Bailin, Ortoli, LLP. From January 1, 2001 to December 31, 2003, he was Counsel to the law firm of Spitzer & Feldman PC. Mr. Sanders also serves as a Director of Helijet International, Inc. and Electrameccanica Vehicles Corp. (NASDAQ:SOLO). Additionally, he has been a director at the American Academy of Dramatic Arts since October 2013 and has been a director of the Bay Street Theater since February 2015. Mr. Sanders received his JD from Cornell University and his BBA from The City College of New York. Mr. Sanders is qualified to serve as a director because of his corporate, securities and international law experience, including working with companies in the life sciences industry.
68
Lourdes Felix, Director
Ms. Felix has served as a member of the Board of the Directors since January 9, 2023. Ms. Felix is an entrepreneur and corporate finance executive with 30 years of combined experience in capital markets, public accounting and in the private sector. She presently serves as Chief Executive Officer, Chief Financial Officer, and Director of BioCorRx Inc, a company focused on addiction treatment solutions and related disorders. She has been with BioCorRx since October 2012. Ms. Felix is one of the founders and President of BioCorRx Pharmaceuticals Inc., a majority owned subsidiary of BioCorRx Inc. Prior to joining BioCorRx, her experience was in the private sector and public accounting. She has expertise in finance, accounting, company-wide operations, budgeting, and internal control principles including GAAP, SEC, and SOX Compliance. She has thorough knowledge of federal and state regulations and has successfully managed and produced SEC regulatory filings. She also has extensive experience in developing and managing financial operations. Lourdes holds a Bachelor of Science degree in Accounting from the University of Phoenix. She continued her education and is an MBA candidate at D’Amore-McKim School of Business, Northeastern University. Ms. Felix is qualified to serve as a director because of her extensive investment and executive level management experience.
Wilbert J. Tauzin II, Director
Wilbert J. Tauzin II has served as a member of the Board of Directors since November 1, 2017. From December 2010 until March 1, 2014, Congressman Tauzin served as Special Legislative Counsel to Alston & Bird LLP. From December 2004 to June 2010, Congressman Tauzin was President and Chief Executive Officer of the Pharmaceutical Research and Manufacturers of America, a trade group that serves as one of the pharmaceutical industry’s top lobbying groups. He served 12.5 terms in the U.S. House of Representatives, representing Louisiana’s 3rd Congressional District. From January 2001 through February 2004, Congressman Tauzin served as Chairman of the House Committee on Energy and Commerce. He also served as a senior member of the House Resources Committee and Deputy Majority Whip. Prior to serving as a member of Congress, Congressman Tauzin was a member of the Louisiana State Legislature, where he served as Chairman of the House Natural Resources Committee and Chief Administration Floor Leader. He served as Lead Independent Director of LHC Group, a publicly traded provider of quality home health care, from 2005 to 2021 and retains the role of Lead Independent Emeritus today. The Congressman also served on the Board of Entergy, a Fortune 500 company. In addition, the Congressman chartered a Louisiana State Savings and Loan Association and Chaired its first Board. He received a Bachelor of Arts Degree from Nicholls State University and a Juris Doctor degree from Louisiana State University. Congressman Tauzin is qualified to serve as a director because of his extensive knowledge of the pharmaceutical industry and his experience as a director of several publicly-traded and privately-held companies.
William B. Stilley, III, Director
William B. Stilley has served as a member of the Board of Directors since July 5, 2018. Mr. Stilley has been the chief executive officer of Purnovate, Inc., a subsidiary of Adial Pharmaceuticals, Inc. (Adial) since January 2021, was chief executive officer of Adial from December 2010 until August 2022, and continues as a member of Adial’s board of directors, which he joined in December 2010. From August 2008 until December 2010, he was the vice president, business development and strategic projects at Clinical Data, Inc. (NASDQ: CLDA). In September 2021, Mr. Stilley was appointed to serve as a member of the board of directors of Sysorex, Inc., where he serves as chair of the audit committee. From February 2002, Mr. Stilley was the COO and CFO of Adenosine Therapeutics, LLC until certain assets of Adenosine Therapeutics were acquired by Clinical Data, Inc. in August 2008. Mr. Stilley has advised both public and private companies on financing and M&A transactions, has been the interim CFO of a public company, the interim Chief Business Officer and then Advisor for Diffusion Pharmaceuticals from September 2015 through March 2018, and the COO and CFO of a number of private companies. Before entering the business community, Mr. Stilley served as Captain in the U.S. Marine Corps. Mr. Stilley has an MBA with honors from the Darden School of Business and a B.S. in Commerce/Marketing from the McIntire School of Commerce at the University of Virginia. He currently serves on the Advisory Board of Virginia BIO, the statewide biotechnology organization. Mr. Stilley is qualified to serve as a director because of his extensive knowledge of the biotechnology industry, significant executive leadership and operational experience, and knowledge of, and experience in, financing and M&A transactions.
69
Tevi Troy, Director
Tevi Troy has served as a member of the Board of Directors since June 4, 2018. Mr. Troy is a former Deputy Secretary of the U.S. Department of Health and Human Services. Dr. Troy is a Senior Fellow at the Bipartisan Policy Center in Washington. He has previously been the founder and CEO of the American Health Policy Institute and a Senior Fellow at Hudson Institute. On August 3, 2007, Dr. Troy was unanimously confirmed by the U.S. Senate as the Deputy Secretary of HHS. As Deputy Secretary, Dr. Troy was the chief operating officer of the largest civilian department in the federal government, with a budget of $716 billion and over 67,000 employees. Dr. Troy has extensive White House experience, having served in several high-level positions over a five-year period, culminating in his service as Deputy Assistant and then Acting Assistant to the President for Domestic Policy. Dr. Troy has held high-level positions on Capitol Hill as well. From 1998 to 2000, Dr. Troy served as the Policy Director for Senator John Ashcroft. From 1996 to 1998, Dr. Troy was Senior Domestic Policy Adviser and later Domestic Policy Director for the House Policy Committee, chaired by Christopher Cox. In addition to his senior level government work and health care expertise, Dr. Troy is also a best-selling presidential historian and the author of five books, including, most recently, “Fight House: Rivalries in the White House from Truman to Trump,” which the Wall Street Journal listed as one of the top political books of 2020. Dr. Troy’s many other affiliations include: contributing editor for Washingtonian magazine; member of the publication committee of National Affairs; member of the Board of Fellows of the Jewish Policy Center; a Senior Fellow at the Potomac Institute; and a member of the Bipartisan Commission on Biodefense. Dr. Troy has a B.S. in Industrial and Labor Relations from Cornell University and an M.A and Ph.D. in American Civilization from the University of Texas at Austin. Dr. Troy is qualified to serve as a director because of his extensive knowledge of the healthcare industry and his significant leadership experience.
Board Composition
Our business and affairs are organized under the direction of our board of directors, which currently consists of nine members. The primary responsibility of our board of directors is to provide oversight, strategic guidance, counseling, and direction to our management team. Our board of directors meets on a regular basis and additionally as required.
A majority of the authorized number of directors constitutes a quorum of the Board of Directors for the transaction of business. The directors must be present at the meeting to constitute a quorum. However, any action required or permitted to be taken by the Board of Directors may be taken without a meeting if all members of the Board of Directors individually or collectively consent in writing to the action.
Director Independence
Our board of directors currently consists of seven members. Our board of directors has determined that William B. Stilley, III, Steven A. Sanders, Tevi Troy, and Lourdes Felix, qualify as independent directors in accordance with the Nasdaq Capital Market (“Nasdaq”) listing requirements.
As required under Nasdaq rules and regulations, our independent directors meet in regularly scheduled executive sessions at which only independent directors are present.
Family Relationships
There are no family relationships among our directors or executive officers.
Board Leadership Structure and Role in Risk Oversight
Our Board of Directors, or the Board, is primarily responsible for overseeing our risk management processes on behalf of our company. The Board receives and reviews periodic reports from management, auditors, legal counsel, and others, as considered appropriate regarding our company’s assessment of risks. In addition, the Board focuses on the most significant risks facing our company and our company’s general risk management strategy, and also ensures that risks undertaken by our company are consistent with the board’s appetite for risk. While the Board oversees our company’s risk management, management is responsible for day-to-day risk management processes. We believe this division of responsibilities is the most effective approach for addressing the risks facing our company and that our board leadership structure supports this approach.
70
Board Committees
Establishment of Board Committees and Adoption of Charters
In November 2018, the Company established a Nominating and Corporate Governance Committee, a Compensation Committee and an Audit Committee (collectively, the “Committees”) and approved and adopted charters to govern each of the Committees.
In connection with the establishment of the Nominating and Corporate Governance Committee, Compensation Committee and Audit Committee, the Board of Directors of the Company appointed members to each such committee. Currently, all three committees are comprised of at least three (3) directors meeting the requirements set forth in each applicable charter. The membership of these three standing committees of the Board of Directors of the Company is as follows:
| Nominating
and Corporate Governance Committee |
Compensation Committee | Audit Committee | ||
| Steven Sanders (Chairman) | Lourdes Felix (Chairwoman) | William Stilley (Chairman) | ||
| Tevi Troy | Steven Sanders | Tevi Troy | ||
| William Stilley | Tevi Troy | Steve Sanders |
Nominating and Corporate Governance Committee
Our board of directors has determined that each of the members of the Nominating and Governance Committee (the “Governance Committee”) are “independent directors” as defined by Nasdaq. The Governance Committee is generally responsible for recommending to our full board of directors’ policies, procedures, and practices designed to help ensure that our corporate governance policies, procedures, and practices continue to assist the board of directors and our management in effectively and efficiently promoting the best interests of our stockholders. The Governance Committee is also responsible for selecting and recommending for approval by our board of directors and our stockholders a slate of director nominees for election at each of our annual meetings of stockholders, and otherwise for determining the board committee members and chairmen, subject to board of directors ratification, as well as recommending to the board director nominees to fill vacancies or new positions on the board of directors or its committees that may occur or be created from time to time, all in accordance with our bylaws and applicable law. The Governance Committee’s principal functions include:
| ● | developing and maintaining our corporate governance policy guidelines; |
| ● | developing and maintaining our codes of conduct and ethics; |
| ● | overseeing the interpretation and enforcement of our Code of Conduct and our Code of Ethics for Chief Executive Officer and Senior Financial and Accounting Officers; |
| ● | evaluating the performance of our board of directors, its committees, and committee chairmen and our directors; and |
| ● | selecting and recommending a slate of director nominees for election at each of our annual meetings of the stockholders and recommending to the board director nominees to fill vacancies or new positions on the board of directors or its committees that may occur from time to time. |
During 2022, the Nominating and Corporate Governance Committee did not meet. The Governance Committee is governed by a written charter approved by our board of directors. A copy of the Governance Committee’s charter is posted on the Company’s website at www.avalon-globocare.com in the “Investors” section of the website. In identifying potential independent board of directors’ candidates with significant senior-level professional experience, the Governance Committee solicits candidates from the board of directors, senior management and others and may engage a search firm in the process. The Governance Committee reviews and narrows the list of candidates and interviews potential nominees. The final candidate is also introduced and interviewed by the board of directors and the lead director if one has been appointed. In general, in considering whether to recommend any particular candidate for inclusion in our board of directors’ slate of recommended director nominees, the Governance Committee will apply the criteria set forth in our corporate governance guidelines. These criteria include the candidate’s integrity, business acumen, commitment to understanding our business and industry, experience, conflicts of interest and the ability to act in the interests of our stockholders. Further, specific consideration is given to, among other things, diversity of background and experience that a candidate would bring to our board of directors. The Governance Committee does not assign specific weights to particular criteria and no particular criterion is a prerequisite for each prospective nominee. We believe that the backgrounds and qualifications of our directors, considered as a group, should provide a composite mix of experience, knowledge and abilities that will allow our board of directors to fulfill its responsibilities. Stockholders may recommend individuals to the Governance Committee for consideration as potential director candidates by submitting their names, together with appropriate biographical information and background materials to our Governance Committee. Assuming that appropriate biographical and background material has been provided on a timely basis, the Governance Committee will evaluate stockholder recommended candidates by following substantially the same process, and applying substantially the same criteria, as it follows for candidates submitted by others.
71
Audit Committee
We have a separately-designated standing Audit Committee established in accordance with Section 3(a)(58)(A) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Our board of directors has determined that the members are all “independent directors” as defined by the rules of Nasdaq applicable to members of an audit committee and Rule 10A-3(b)(i) under the Exchange Act. In addition, Mr. Stilley is an “audit committee financial expert” as defined in Item 407(d)(5) of Regulation S-K and demonstrates “financial sophistication” as defined by the rules of The NASDAQ Stock Market, Inc. The Audit Committee is appointed by our board of directors to assist our board of directors in monitoring (1) the integrity of our financial statements, (2) our compliance with legal and regulatory requirements, and (3) the independence and performance of our internal and external auditors. The Audit Committee’s principal functions include:
| ● | reviewing our annual audited financial statements with management and our independent auditors, including major issues regarding accounting and auditing principles and practices and financial reporting that could significantly affect our financial statements; |
| ● | reviewing our quarterly financial statements with management and our independent auditor prior to the filing of our Quarterly Reports on Form 10-Q, including the results of the independent auditors’ reviews of the quarterly financial statements; |
| ● | recommending to the board of directors the appointment of, and continued evaluation of the performance of, our independent auditor; |
| ● | approving the fees to be paid to our independent auditor for audit services and approving the retention of our independent auditor for non-audit services and all fees for such services; |
| ● | reviewing periodic reports from our independent auditor regarding our auditor’s independence, including discussion of such reports with the auditor; |
| ● | reviewing the adequacy of our overall control environment, including internal financial controls and disclosure controls and procedures; and |
| ● | reviewing with our management and legal counsel legal matters that may have a material impact on our financial statements or our compliance policies and any material reports or inquiries received from regulators or governmental agencies. |
During the year ended December 31, 2022, the audit committee met four times. A copy of the Audit Committee’s charter is posted on the Company’s website at www.avalon-globocare.com in the “Investors” section of the website.
Meetings may be held from time to time to consider matters for which approval of our Board of Directors is desirable or is required by law.
Compensation Committee
Our compensation committee consists of Lourdes Felix, Steven Sanders and Tevi Troy. Our board of directors has determined that each of the members are an “independent director” as defined by the Nasdaq rules applicable to members of a compensation committee. The Compensation Committee is responsible for establishing the compensation of our senior management, including salaries, bonuses, termination arrangements, and other executive officer benefits as well as director compensation. The Compensation Committee also administers our equity incentive plans. During the year ended December 31, 2022, the Compensation Committee did not meet. The Compensation Committee is governed by a written charter approved by the board of directors. A copy of the Compensation Committee’s charter is posted on the Company’s website at www.avalon-globocare.com in the “Investors” section of the website. The Compensation Committee works with the Chairman of the Board and Chief Executive Officer and reviews and approves compensation decisions regarding senior management including compensation levels and equity incentive awards. The Compensation Committee also approves employment and compensation agreements with our key personnel and directors. The Compensation Committee has the power and authority to conduct or authorize studies, retain independent consultants, accountants or others, and obtain unrestricted access to management, our internal auditors, human resources and accounting employees and all information relevant to its responsibilities.
The responsibilities of the Compensation Committee, as stated in its charter, include the following:
| ● | review and approve the Company’s compensation guidelines and structure; |
| ● | review and approve on an annual basis the corporate goals and objectives with respect to compensation for the Chief Executive Officer; |
72
| ● | review and approve on an annual basis the evaluation process and compensation structure for the Company’s other officers, including salary, bonus, incentive and equity compensation; and |
| ● | periodically review and make recommendations to the Board of Directors regarding the compensation of non-management directors. |
The Compensation Committee is responsible for developing the executive compensation philosophy and reviewing and recommending to the Board of Directors for approval all compensation policies and compensation programs for the executive team.
Compensation Committee Interlocks and Insider Participation
None of our executive officers currently serves, or in the past year has served, as a member of the board of directors or compensation committee of any entity that has one or more executive officers on our board of directors or compensation committee.
Code of Ethics
We have a code of ethics that applies to all of our employees, including our principal executive officer, principal financial officer and principal accounting officer, and the Board. A copy of this code is available in our employee handbook and under the “About Us – Code of Conduct” section of our website at www.avalon-globocare.com. In addition, we intend to post on our website all disclosures that are required by law or the listing standards of our applicable trading market concerning any amendments to, or waivers from, any provision of the code. The reference to our website address does not constitute incorporation by reference of the information contained at or available through our website, and you should not consider it to be a part of this report.
Indemnification of Directors and Officers
Our directors and executive officers are indemnified as provided by the Delaware law and our Bylaws. These provisions state that our directors may cause us to indemnify a director or former director against all costs, charges and expenses, including an amount paid to settle an action or satisfy a judgment, actually and reasonably incurred by him or her as a result of him or her acting as a director. The indemnification of costs can include an amount paid to settle an action or satisfy a judgment. Such indemnification is at the discretion of our board of directors and is subject to the Securities and Exchange Commission’s policy regarding indemnification.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, or otherwise. We have been advised that in the opinion of the Securities and Exchange Commission, such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
Delinquent Section 16(a) Reports
Section 16(a) of the Exchange Act requires the Company’s executive officers, directors, and persons who beneficially own more than ten percent of a registered class of the Company’s equity securities, to file with the SEC initial reports of ownership and reports of changes in ownership of the Company’s common stock. Such officers, directors, and persons are required by SEC regulation to furnish the Company with copies of all Section 16(a) forms that they file with the SEC.
To our knowledge, based solely on review of the copies of such reports and amendments to such reports with respect to the year ended December 31, 2022 filed with the SEC, all required Section 16 reports under the Exchange Act for our directors, executive officers, principal accounting officer and beneficial owners of greater than 10% of our common stock were filed on a timely basis during the year ended December 31, 2022.
73
ITEM 11. EXECUTIVE COMPENSATION
Executive Officers’ Compensation
The following table sets forth information concerning all cash and non-cash compensation awarded to, earned by or paid to our Chief Executive Officer, Chief Financial Officer and Chief Operation Officer during the last two (2) years. No other executive officer received compensation in excess of $100,000 during the fiscal year ended December 31, 2022.
Summary Annual Compensation Table
| Name and Principal Position | Fiscal Year | Salary | Stock Award | Option Awards | Non-Equity Incentive Plan Compensation | Change in Pension Value and Non- Qualified Deferred Compensation Earnings | All Other Compensation | Total | ||||||||||||||||||||
| ($) | ($) | ($) | ($) | ($) | ($) | ($) | ||||||||||||||||||||||
| Dr. David Jin | 2022 | 360,000 | - | - | - | - | - | 360,000 | ||||||||||||||||||||
| CEO | 2021 | 360,000 | - | - | - | - | - | 360,000 | ||||||||||||||||||||
| Luisa Ingargiola | 2022 | 350,000 | - | - | - | - | - | 350,000 | ||||||||||||||||||||
| CFO | 2021 | 350,000 | - | - | - | - | - | 350,000 | ||||||||||||||||||||
| Meng Li | 2022 | 340,000 | - | - | - | - | - | 340,000 | ||||||||||||||||||||
| COO | 2021 | 340,000 | - | - | - | - | - | 340,000 | ||||||||||||||||||||
Employment Agreements
David Jin
On December 1, 2016, the Company entered into an Executive Employment Agreement with David Jin, the Company’s CEO and President. Pursuant to the agreement, Mr. Jin was employed as President and Chief Executive Officer of the Company which agreement had a term initially through November 30, 2017 unless earlier terminated pursuant to the terms of the agreement. On February 20, 2020, the Company entered into a Letter Agreement with Dr. Jin pursuant to which the term of Dr. Jin’s Executive Employment Agreement was extended an additional three years.
During the term of the agreement, Mr. Jin is entitled to a base salary and will be eligible for a discretionary performance bonus, equity awards and to participate in employee benefits plans as the Company may institute from time to time at the discretion of the Company’s Board of Directors. On January 3, 2019, the Company entered into a Letter Agreement with Dr. Jin, pursuant to which his annual base salary set forth in his employment agreement was increased to $360,000 effective January 1, 2019. Pursuant to the agreement, Mr. Jin may be terminated for “cause” as defined and Mr. Jin may resign for “good reason” as defined. In the event Mr. Jin is terminated without cause or resigns for good reason, the Company will be required to pay Mr. Jin all accrued salary and bonuses, reimbursement for all business expenses and Mr. Jin’s salary for one year. In the event Mr. Jin is terminated with cause, resigns without good reason, dies or is disabled, the Company will be required to pay Mr. Jin all accrued salary and bonuses and reimbursement for all business expenses. Under the agreement Mr. Jin is subject to confidentiality, non-compete and non-solicitation restrictions.
74
Meng Li
On January 11, 2017, Avalon Shanghai entered into an Executive Employment Agreement with Meng Li, the Company’s COO and Secretary. Pursuant to the agreement, Ms. Li was employed as Chief Operating Officer and President of Avalon Shanghai initially through November 30, 2019, unless earlier terminated pursuant to the terms of the agreement. On February 20, 2020, the Company entered into a Letter Agreement with Meng Li pursuant to which the term of Ms. Li’s Executive Employment Agreement entered between the Company’ subsidiary and Ms. Li dated January 11, 2017 was extended an additional three years.
During the term of the agreement, Ms. Li is be entitled to a base salary and will be eligible for a discretionary performance bonus, equity awards and to participate in employee benefits plans as the Avalon Shanghai may institute from time to time at the discretion of its Board of Directors. On January 3, 2019, the Company entered into a Letter Agreement with Ms. Li, pursuant to which her annual base salary set forth in her employment agreement was increased to $340,000 effective January 1, 2019. Pursuant to the agreement, Ms. Li may be terminated for “cause” as defined and Ms. Li may resign for “good reason” as defined. In the event Ms. Li is terminated without cause or resigns for good reason, Avalon Shanghai will be required to pay Ms. Li all accrued salary and bonuses, reimbursement for all business expenses and Ms. Li’s salary for one year. In the event Ms. Li is terminated with cause, resigns without good reason, dies or is disabled, Avalon Shanghai will be required to pay Ms. Li all accrued salary and bonuses and reimbursement for all business expenses. Under the agreement Ms. Li is subject to confidentiality, non-compete and non-solicitation restrictions.
Luisa Ingargiola
On February 21, 2017, Ms. Ingargiola and the Company entered into an Executive Retention Agreement effective February 9, 2017 pursuant to which Ms. Ingargiola agreed to serve as Chief Financial Officer in consideration of an annual salary. On January 3, 2019, the Company entered into a Letter Agreement with Ms. Ingargiola, pursuant to which her annual base salary set forth in her employment agreement was increased to $350,000 effective January 1, 2019.
The employment of Ms. Ingargiola is at will and may be terminated at any time, with or without formal cause. Pursuant to the terms of executive retention agreement with Ms. Ingargiola, the Company has agreed to provide specified severance and bonus amounts and to accelerate the vesting on their equity awards upon termination upon a change of control or an involuntary termination, as each term is defined in the agreements.
In the event of a termination upon a change of control, Ms. Ingargiola is entitled to receive an amount equal to 12 months of her base salary and the target bonus then in effect for the executive officer for the year in which such termination occurs, such bonus payment to be pro-rated to reflect the full number of months the executive remained in the Company’s employ. In addition, the vesting on any stock option held by the executive officer will be accelerated in full. At the election of the executive officer, the Company will also continue to provide health related employee insurance coverage for twelve months, at the Company’s expense.
75
In the event of an involuntary termination, Ms. Ingargiola is entitled to receive an amount equal to six months of her base salary and the target bonus then in effect for the executive officer for the six months in which such termination occurs, such bonus payment to be pro-rated to reflect the full number of months the executive remained in the Company’s employ. Such payment will be increased to 12 months upon the one-year anniversary of the retention agreement. In addition, the vesting on any stock option held by the executive officer will be accelerated in full. At the election of the executive officer, the Company will also continue to provide health related employee insurance coverage for twelve months, at the Company’s expense.
Option Exercises and Stock Vested
There were no options exercised by our executive officers or stock vested to our executive officers during the year ended December 31, 2022.
Outstanding Equity Awards
The following table sets forth information with respect to the outstanding equity awards of our principal executive officers and principal financial officer during 2022, and each person who served as an executive officer of the Company as of December 31, 2022:
| Outstanding Equity Awards | ||||||||||||||||||||||||||||||||||||
| Option Awards | Stock Awards | |||||||||||||||||||||||||||||||||||
| Name and principal position | Number
of (#) |
Number
of (#) |
Equity incentive plan awards: Number of securities underlying unexercised options (#) |
Options ($) |
Option expiration Date |
Number
of shares or units of stock that have not vested (#) |
Market value of shares or units of stock that have not vested ($) |
Equity incentive plan awards: Number of unearned shares, units or other rights that have not vested (#) |
Equity incentive plan awards: Market or payout value of unearned shares, units or other rights that have not vested ($) |
|||||||||||||||||||||||||||
| Luisa Ingargiola, CFO | 240,000 | - | 240,000 | 5.0 | 2/8/2027 | - | - | - | - | |||||||||||||||||||||||||||
| 15.2 | 2/18/2030 | |||||||||||||||||||||||||||||||||||
| David Jin, CEO | 55,000 | - | 55,000 | 20.0 | 1/2/2024 | - | - | - | - | |||||||||||||||||||||||||||
| 15.2 | 2/18/2030 | |||||||||||||||||||||||||||||||||||
| Meng Li, COO | 45,000 | - | 45,000 | 20.0 | 1/2/2024 | - | - | - | - | |||||||||||||||||||||||||||
| 15.2 | 2/18/2030 | |||||||||||||||||||||||||||||||||||
76
No Pension Benefits
The Company does not maintain any plan that provides for payments or other benefits to its executive officers at, following or in connection with retirement and including, without limitation, any tax-qualified defined benefit plans or supplemental executive retirement plans.
No Nonqualified Deferred Compensation
The Company does not maintain any defined contribution or other plan that provides for the deferral of compensation on a basis that is not tax-qualified.
Director Compensation
Name |
Fees |
Stock |
Option |
Non-equity | Change
in $ |
All
Other |
Total $ | |||||||||||||||||||||
| Yue (Charles) Li (1) | 60,000 | - | 31,667 | - | - | - | 91,667 | |||||||||||||||||||||
| Yancen Lu (2) | 70,000 | - | 31,667 | - | - | - | 101,667 | |||||||||||||||||||||
| Wilbert Tauzin (3) | - | - | 94,890 | - | - | - | 94,890 | |||||||||||||||||||||
| Wenzhao Lu | 100,000 | - | - | - | - | - | 100,000 | |||||||||||||||||||||
| David Jin | - | - | - | - | - | - | - | |||||||||||||||||||||
| Meng Li (4) | - | - | - | - | - | - | - | |||||||||||||||||||||
| Steven Sanders (5) | 70,000 | - | 55,274 | - | - | - | 125,274 | |||||||||||||||||||||
| Tevi Troy (6) | 60,000 | - | 55,274 | - | - | - | 115,274 | |||||||||||||||||||||
| William Stilley (7) | 70,000 | - | 55,274 | - | - | - | 125,274 | |||||||||||||||||||||
| (1) | Mr. Li’s 2022 compensation consisted of cash of $60,000 and 8,000 options vested and valued at $31,667. Mr. Li resigned as a director on December 30, 2022. |
| (2) | Mr. Lu’s 2022 compensation consisted of cash of $70,000 and 8,000 options vested and valued at $31,667. Mr. Lu resigned as a director on December 30, 2022. |
| (3) | Mr. Tauzin’s 2022 compensation consisted of 200,000 options vested and valued at $94,890. |
| (4) | Ms. Li resigned as a director on December 30, 2022. |
| (5) | Mr. Sanders’s 2022 compensation consisted of cash of $70,000 and 8,000 options vested and valued at $55,274. |
| (6) | Mr. Troy’s 2022 compensation consisted of cash of $60,000 and 8,000 options vested and valued at $55,274. |
| (7) | Mr. Stilley’s 2022 compensation consisted of cash of $70,000 and 8,000 options vested and valued at $55,274. |
77
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. In accordance with SEC rules, shares of our common stock which may be acquired upon exercise of stock options or warrants which are currently exercisable or which become exercisable within 60 days of the date of the applicable table below are deemed beneficially owned by the holders of such options and warrants and are deemed outstanding for the purpose of computing the percentage of ownership of such person, but are not treated as outstanding for the purpose of computing the percentage of ownership of any other person. Subject to community property laws, where applicable, the persons or entities named in the tables below have sole voting and investment power with respect to all shares of our common stock indicated as beneficially owned by them.
The following table sets forth certain information, as of March 29, 2023 with respect to the beneficial ownership of the outstanding common stock by (i) any holder of more than five (5%) percent; (ii) each of our executive officers and directors; and (iii) our directors and executive officers as a group. The numbers below reflect a 1:10 reverse stock split implemented on January 5, 2023. Except as otherwise indicated, each of the stockholders listed below has sole voting and investment power over the shares beneficially owned.
| Name of Beneficial Owner (1) | Common
Stock Beneficially Owned | Percentage
of Common Stock (2) | ||||||
| Wenzhao Lu* (3) | 3,733,788 | 33.6 | % | |||||
| David Jin, MD, PhD* (4) | 1,600,000 | 14.4 | % | |||||
| Meng Li* (5) | 560,000 | 5.0 | % | |||||
| Luisa Ingargiola* (6) | 240,000 | 2.2 | % | |||||
| Steven A. Sanders* (7) | 33,000 | ** | ||||||
| Wilbert J. Tauzin II* (8) | 65,000 | ** | ||||||
| William B. Stilley III* (9) | 33,000 | ** | ||||||
| Tevi Troy* (10) | 33,000 | ** | ||||||
| Lourdes Felix* (11) | 3,803 | ** | ||||||
| All officers and directors as a group (9 persons) | 6,301,591 | 56.8 | % | |||||
| Shareholder owning 5% or more: | ||||||||
| FSUNSHINE TRADING PTE LTD | 573,646 | 5.2 | % | |||||
| * | Officer and/or director of our company. |
| ** | Less than 1.0%. |
| (1) | Except as otherwise indicated, the address of each beneficial owner is c/o Avalon GloboCare Corp., 4400 Route 9 South, Suite 3100, Freehold, New Jersey 07728. |
| (2) | Applicable percentage ownership is based on 10,164,307 shares of common stock outstanding as of March 29, 2023, together with securities exercisable or convertible into shares of common stock within 60 days of March 29, 2023 for each stockholder. Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission and generally includes voting or investment power with respect to securities. Shares of common stock that are currently exercisable or exercisable within 60 days of March 29, 2023 are deemed to be beneficially owned by the person holding such securities for the purpose of computing the percentage of ownership of such person, but are not treated as outstanding for the purpose of computing the percentage ownership of any other person. |
| (3) | Wenzhao Lu holds (i) 3,583,788 shares of common stock and (ii) 150,000 vested options to acquire 150,000 shares of common stock of our company. |
| (4) | David Jin holds (i) 1,545,000 shares of common stock and (ii) 55,000 vested options to acquire 55,000 shares of common stock of our company. |
78
| (5) | Meng Li holds (i) 515,000 shares of common stock and (ii) 45,000 vested options to acquire 45,000 shares of common stock of our company. |
| (6) | Represents 240,000 vested options to acquire 240,000 shares of common stock of our company. |
| (7) | Represents stock option to acquire 33,000 shares of common stock of our company, which included 2,000 shares to be vested within 60 days. |
| (8) | Represents stock option to acquire 65,000 shares of common stock of our company, which included 1,000 shares to be vested within 60 days. |
| (9) | Represents stock option to acquire33,000 shares of common stock of our company, which included 2,000 shares to be vested within 60 days. |
| (10) | Represents stock option to acquire 33,000 shares of common stock of our company, which included 2,000 shares to be vested within 60 days. |
| (11) | Represents stock option to acquire 3,803 shares of common stock of our company. |
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Rental Revenue from Related Party and Rent Receivable – Related Party
The Company leases space of its commercial real property located in New Jersey to a company, D.P. Capital Investments LLC, which is controlled by Wenzhao Lu, the Company’s largest shareholder and chairman of the Board of Directors. The term of the related party lease agreement is five years commencing on May 1, 2021 and will expire on April 30, 2026.
For the years ended December 31, 2022 and 2021, the related party rental revenue amounted to $50,400 and $33,600, respectively, and has been included in real property rental on the accompanying consolidated statements of operations and comprehensive loss.
The related party rent receivable totaled $74,100 and $33,600, respectively, and no allowance for doubtful accounts was deemed to be required on rent receivable – related party at December 31, 2022 and 2021.
Medical Related Consulting Services Revenue from Related Party
During the years ended December 31, 2022 and 2021, medical related consulting services revenue from related party was as follows:
| Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Medical related consulting services provided to: | ||||||||
| Hebei Daopei * | $ | - | $ | 187,412 | ||||
| $ | - | $ | 187,412 | |||||
| * | Hebei Daopei is a subsidiary of an entity whose chairman is Wenzhao Lu, the largest shareholder of the Company. |
Services Provided by Related Party
From time to time, Wilbert Tauzin, a director of the Company, and his son provide consulting services to the Company. As compensation for professional services provided, the Company recognized consulting expenses of $144,064 and $216,169 for the years ended December 31, 2022 and 2021, respectively, which have been included in professional fees on the accompanying consolidated statements of operations and comprehensive loss.
79
Accrued Liabilities and Other Payables – Related Parties
In 2017, the Company acquired Beijing Genexosome for a cash payment of $450,000. As of December 31, 2022 and 2021, the unpaid acquisition consideration of $100,000, was payable to Dr. Yu Zhou, former director and former co-chief executive officer and 40% owner of Genexosome, and has been included in accrued liabilities and other payables – related parties on the accompanying consolidated balance sheets.
As of December 31, 2022 and 2021, $0 and $368,433 of accrued and unpaid interest related to borrowings from Wenzhao Lu, the Company’s largest shareholder and chairman of the Board of Directors, respectively, have been included in accrued liabilities and other payables – related parties on the accompanying consolidated balance sheets.
Borrowings from Related Party
Promissory Note
On March 18, 2019, the Company issued Wenzhao Lu, the Company’s largest shareholder and Chairman of the Board of Directors, a Promissory Note in the principal amount of $1,000,000 (“Promissory Note”) in consideration of cash in the amount of $1,000,000. The Promissory Note accrues interest at the rate of 5% per annum and matures March 19, 2022. In March 2022, the Company and Wenzhao Lu entered into a Loan Extension and Modification Agreement (the “Extension”) to extend the maturity date to March 19, 2024.The Company repaid principal of $410,000, $200,000 and $390,000 in the third quarter of 2019, second quarter of 2020 and second quarter of 2022, respectively. As of December 31, 2022 and 2021, the outstanding principal balance was $0 and $390,000, respectively.
Line of Credit
On August 29, 2019, the Company entered into a Line of Credit Agreement (the “Line of Credit Agreement”) providing the Company with a $20 million line of credit (the “Line of Credit”) from Wenzhao Lu (the “Lender”), the largest shareholder and Chairman of the Board of Directors of the Company. The Line of Credit allows the Company to request loans thereunder and to use the proceeds of such loans for working capital and operating expense purposes until the facility matures on December 31, 2024. The loans are unsecured and are not convertible into equity of the Company. Loans drawn under the Line of Credit bears interest at an annual rate of 5% and each individual loan will be payable three years from the date of issuance. The Company has a right to draw down on the line of credit and not at the discretion of the related party Lender. The Company may, at its option, prepay any borrowings under the Line of Credit, in whole or in part at any time prior to maturity, without premium or penalty. The Line of Credit Agreement includes customary events of default. If any such event of default occurs, the Lender may declare all outstanding loans under the Line of Credit to be due and payable immediately.
In the years ended December 31, 2022 and 2021, activity recorded for the Line of Credit is summarized in the following table:
| Outstanding principal under the Line of Credit at January 1, 2021 | $ | 3,200,000 | ||
| Draw down from Line of Credit | 2,550,262 | |||
| Settlement of Line of Credit in shares | (3,000,000 | ) | ||
| Outstanding principal under the Line of Credit at December 31, 2021 | 2,750,262 | |||
| Draw down from Line of Credit | 100,000 | |||
| Repayment of Line of Credit | (410,000 | ) | ||
| Settlement of Line of Credit in shares | (2,440,262 | ) | ||
| Outstanding principal under the Line of Credit at December 31, 2022 | $ | - |
For the years ended December 31, 2022 and 2021, the interest expense related to above borrowings amounted to $79,898 and $200,477, respectively, and has been reflected as interest expense – related party on the accompanying consolidated statements of operations and comprehensive loss.
80
As of December 31, 2022 and 2021, the related accrued and unpaid interest for above borrowings was $0 and $368,433, respectively, and has been included in accrued liabilities and other payables – related parties on the accompanying consolidated balance sheets.
Common Shares Sold to Related Party for Cash
On August 5, 2022, the Company sold 44,872 shares of its common stock at a purchase price of $7.8 per share, the fair market value on transaction date, to Wenzhao Lu pursuant to a subscription agreement. The Company received proceeds of $350,000.
Series A Convertible Preferred Stock Sold to Related Party for Cash
On December 14, 2022, the Company entered into a Securities Purchase Agreement with Wenzhao Lu, the Company’s Chairman of the Board, pursuant to which the Company sold to Mr. Lu 4,000 shares of its Series A Preferred Stock, stated value $1,000, for the gross proceeds of $4,000,000.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
Marcum LLP served as our independent auditors for the years ended December 31, 2022 and 2021.
Aggregate fees billed to the Company for professional services rendered by Marcum LLP during the last two years were as follows:
| Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Audit Fees | $ | 196,473 | $ | 223,229 | ||||
| Audit Related Fees | - | - | ||||||
| Tax Fees | - | - | ||||||
| All Other Fees | - | - | ||||||
| Totals | $ | 196,473 | $ | 223,229 | ||||
AUDIT FEES. Consists of fees billed for professional services rendered for the audit of our annual consolidated financial statements, review of the Form 10-K, and review of the interim consolidated financial statements included in quarterly reports, and services that are normally provided by our independent auditors in connection with statutory and regulatory filings or engagements, including registration statements.
AUDIT-RELATED FEES. Consists of fees billed for assurance and related services that are reasonably related to the performance of the audit and or review of our consolidated financial statements and are not reported under “Audit Fees”, such as audits and reviews in connection with acquisitions.
TAX FEES. Consists of fees billed for professional services for tax compliance, tax advice and tax planning.
ALL OTHER FEES. Consists of fees for products and services other than the services reported above. There were no management consulting services provided in 2022 or 2021.
POLICY ON AUDIT COMMITTEE PRE-APPROVAL OF AUDIT AND PERMISSIBLE NON-AUDIT SERVICES OF INDEPENDENT AUDITORS
The current policy of the directors, acting as the audit committee, is to approve the appointment of the principal auditing firm and any permissible audit-related services. The audit and audit related fees include fees for the annual audit of the financial statements and review of financial statements included in Form 10-Q filings. Fees charged by the auditor were approved by the Board with engagement letters signed by the audit committee chairman.
The Audit Committee is responsible for the pre-approval of audit and permitted non-audit services to be performed by the Company’s independent auditor. The Audit Committee will, on an annual basis, consider and, if appropriate, approve the provision of audit and non-audit services by the auditor. Thereafter, the Audit Committee will, as necessary, consider and, if appropriate, approve the provision of additional audit and non-audit services by the auditor which are not encompassed by the Audit Committee’s annual pre-approval and are not prohibited by law. The Audit Committee has delegated to the Chair of the Audit Committee the authority to pre-approve, on a case-by-case basis, non-audit services to be performed by the auditor. The Audit Committee has approved all audit and permitted non-audit services performed by the auditor for the year ended December 31, 2022.
81
PART IV
ITEM 15. EXHIBITS
82
83
84
85
86
87
| * | Filed herewith |
| ** | This certification will not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or the Exchange Act, or otherwise subject to the liability of that section. Such certification will not be deemed to be incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act, except to the extent specifically incorporated by reference into such filing. |
| † | Management contract or compensatory plan or arrangement. |
ITEM 16. FORM 10-K SUMMARY.
None.
88
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities and Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| AVALON GLOBOCARE CORP. | ||
| Dated: March 30, 2023 | By: | /s/ David K. Jin |
| Name: | David K. Jin | |
| Title: | Chief Executive Officer, President and Director (Principal Executive Officer) | |
| Dated: March 30, 2023 | By: | /s/ Luisa Ingargiola |
| Name: | Luisa Ingargiola | |
| Title: | Chief Financial Officer (Principal Financial and Accounting Officer) | |
In accordance with the Exchange Act, this report has been signed below by the following persons on March 30, 2023, on behalf of the registrant and in the capacities indicated.
| Signature | Title | |
| /s/ David K. Jin | Chief Executive Officer, President and Director | |
| David K. Jin | (Principal Executive Officer) | |
| /s/ Luisa Ingargolia | Chief Financial Officer | |
| Luisa Ingargolia | (Principal Financial and Accounting Officer) | |
| /s/ Wenzhao Lu | Chairman of the Board of Directors | |
| Wenzhao Lu | ||
| /s/ Meng Li | Chief Operating Officer and Secretary | |
| Meng Li | ||
| /s/ Steven A. Sanders | Director | |
| Steven A. Sanders | ||
| /s/ Lourdes Felix | Director | |
| Lourdes Felix | ||
| /s/ Wilbert J. Tauzin II | Director | |
| Wilbert J. Tauzin II | ||
| /s/ William B. Stilley III | Director | |
| William B. Stilley III | ||
| /s/ Tevi Troy | Director | |
| Tevi Troy |
89
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022 and 2021
CONTENTS
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors of
Avalon GloboCare Corp.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Avalon GloboCare Corp. (the “Company”) as of December 31, 2022 and 2021, and the related consolidated statements of operations and comprehensive loss, changes in equity and cash flows for each of the two years in the period ended December 31, 2022, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022 and 2021, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2022, in conformity with accounting principles generally accepted in the United States of America.
Explanatory Paragraph – Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As more fully described in Note 2 the Company has a significant working capital deficiency, has incurred significant losses and needs to raise additional funds to meet its obligations and sustain its operations. These conditions raise substantial doubt about the Company's ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. We determined that there are no critical audit matters.
/s/ Marcum llp
We have served as the Company’s auditor since 2019.
March 30, 2023
F-2
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
| December 31, | ||||||||
| 2022 | 2021 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash | $ | $ | ||||||
| Rent receivable | ||||||||
| Rent receivable - related party | ||||||||
| Other current assets | ||||||||
| Total Current Assets | ||||||||
| NON-CURRENT ASSETS: | ||||||||
| Operating lease right-of-use assets, net | ||||||||
| Property and equipment, net | ||||||||
| Investment in real estate, net | ||||||||
| Equity method investment | ||||||||
| Advances for equity interest purchase | - | |||||||
| Other non-current assets | ||||||||
| Total Non-current Assets | ||||||||
| Total Assets | $ | $ | ||||||
| LIABILITIES AND EQUITY | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Accrued professional fees | $ | $ | ||||||
| Accrued research and development fees | ||||||||
| Accrued payroll liability and directors’ compensation | ||||||||
| Accrued litigation settlement | ||||||||
| Accrued liabilities and other payables | ||||||||
| Accrued liabilities and other payables - related parties | ||||||||
| Operating lease obligation | ||||||||
| Note payable - related party | ||||||||
| Total Current Liabilities | ||||||||
| NON-CURRENT LIABILITIES: | ||||||||
| Operating lease obligation - noncurrent portion | ||||||||
| Accrued litigation settlement - noncurrent portion | ||||||||
| Note payable, net | ||||||||
| Loan payable - related party | ||||||||
| Total Non-current Liabilities | ||||||||
| Total Liabilities | ||||||||
| Commitments and Contingencies (Note 20) | ||||||||
| EQUITY: | ||||||||
| Preferred stock, $0.0001 par value; 10,000,000 shares authorized; | ||||||||
| Series B Convertible Preferred Stock, $ | ||||||||
| Common stock, $ | ||||||||
| Additional paid-in capital | ||||||||
| Less: common stock held in treasury, at cost; | ||||||||
| ( | ) | ( | ) | |||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| Statutory reserve | ||||||||
| Accumulated other comprehensive loss - foreign currency translation adjustment | ( | ) | ( | ) | ||||
| Total Avalon GloboCare Corp. stockholders’ equity | ||||||||
| Non-controlling interest | ||||||||
| Total Equity | ||||||||
| Total Liabilities and Equity | $ | $ | ||||||
See accompanying notes to the consolidated financial statements.
F-3
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
| For the Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| REVENUES | ||||||||
| Real property rental | $ | $ | ||||||
| Medical related consulting services - related party | ||||||||
| Total Revenues | ||||||||
| COSTS AND EXPENSES | ||||||||
| Real property operating expenses | ||||||||
| Medical related consulting services - related party | - | |||||||
| Total Costs and Expenses | ||||||||
| GROSS PROFIT | ||||||||
| Real property operating income | ||||||||
| Gross profit from medical related consulting services | ||||||||
| Total Gross Profit | ||||||||
| OTHER OPERATING EXPENSES: | ||||||||
| Advertising and marketing | ||||||||
| Professional fees | ||||||||
| Compensation and related benefits | ||||||||
| Research and development expenses | ||||||||
| Litigation settlement | ||||||||
| Other general and administrative | ||||||||
| Total Other Operating Expenses | ||||||||
| LOSS FROM OPERATIONS | ( | ) | ( | ) | ||||
| OTHER (EXPENSE) INCOME | ||||||||
| Interest expense- amortization of debt discount and debt issuance cost | ( | ) | ||||||
| Interest expense- other | ( | ) | ||||||
| Interest expense - related party | ( | ) | ( | ) | ||||
| Conversion inducement expense | ( | ) | ||||||
| Loss from equity method investment | ( | ) | ( | ) | ||||
| Change in fair value of derivative liability | ||||||||
| Other income | ||||||||
| Total Other Expense, net | ( | ) | ( | ) | ||||
| LOSS BEFORE INCOME TAXES | ( | ) | ( | ) | ||||
| INCOME TAXES | ||||||||
| NET LOSS | $ | ( | ) | $ | ( | ) | ||
| LESS: NET LOSS ATTRIBUTABLE TO NON-CONTROLLING INTEREST | ||||||||
| NET LOSS ATTRIBUTABLE TO AVALON GLOBOCARE CORP. COMMON SHAREHOLDERS | $ | ( | ) | $ | ( | ) | ||
| COMPREHENSIVE LOSS: | ||||||||
| NET LOSS ATTRIBUTABLE TO AVALON GLOBOCARE CORP. COMMON SHAREHOLDERS | $ | ( | ) | $ | ( | ) | ||
| OTHER COMPREHENSIVE (LOSS) INCOME | ||||||||
| Unrealized foreign currency translation (loss) gain | ( | ) | ||||||
| COMPREHENSIVE LOSS | ( | ) | ( | ) | ||||
| LESS: COMPREHENSIVE LOSS ATTRIBUTABLE TO NON-CONTROLLING INTEREST | ||||||||
| COMPREHENSIVE LOSS ATTRIBUTABLE TO AVALON GLOBOCARE CORP. COMMON SHAREHOLDERS | $ | ( | ) | $ | ( | ) | ||
| NET LOSS PER COMMON SHARE ATTRIBUTABLE TO AVALON GLOBOCARE CORP. COMMON SHAREHOLDERS: | ||||||||
| $ | ( | ) | $ | ( | ) | |||
| WEIGHTED AVERAGE COMMON SHARES OUTSTANDING: | ||||||||
See accompanying notes to the consolidated financial statements.
F-4
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
For the Years Ended December 31, 2022 and 2021
| Avalon GloboCare Corp. Stockholders’ Equity | ||||||||||||||||||||||||||||||||||||||||||||||||
| Series A Preferred Stock | Common Stock | Treasury Stock | Accumulated | |||||||||||||||||||||||||||||||||||||||||||||
| Number of | Number of | Additional Paid-in | Number of | Accumulated | Statutory | Other Comprehensive | Non- controlling | Total | ||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Shares | Amount | Deficit | Reserve | Loss | Interest | Equity | |||||||||||||||||||||||||||||||||||||
| Balance, January 1, 2021 | $ | $ | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | $ | ( | ) | $ | $ | |||||||||||||||||||||||||||||||
| Sale of common stock, net | ||||||||||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock for settlement of accrued professional fees | ||||||||||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock for settlement of loan payable - related party | - | - | ||||||||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock for services | ||||||||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation | - | - | - | |||||||||||||||||||||||||||||||||||||||||||||
| Foreign currency translation adjustment | - | - | - | |||||||||||||||||||||||||||||||||||||||||||||
| Net loss for the year | - | - | - | ( | ) | ( | ) | |||||||||||||||||||||||||||||||||||||||||
| Balance, December 31, 2021 | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||||||||||||||||
| Sale of common stock, net | ||||||||||||||||||||||||||||||||||||||||||||||||
| Warrants issued with convertible debt offering | - | - | - | |||||||||||||||||||||||||||||||||||||||||||||
| Conversion of convertible note payable and accrued interest into common stock | ||||||||||||||||||||||||||||||||||||||||||||||||
| Reclassification of derivative liability to equity | - | - | - | |||||||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock for settlement of loan payable and accrued interest - related party | ||||||||||||||||||||||||||||||||||||||||||||||||
| Sale of common stock - related party | ||||||||||||||||||||||||||||||||||||||||||||||||
| Sale of Series A Convertible Preferred Stock | ||||||||||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock for services | ||||||||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation | - | - | - | |||||||||||||||||||||||||||||||||||||||||||||
| Shares issued for adjustments for 1:10 reverse split | - | - | ( | ) | - | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||||||||||
| Foreign currency translation adjustment | - | - | - | ( | ) | ( | ) | |||||||||||||||||||||||||||||||||||||||||
| Net loss for the year | - | - | - | ( | ) | ( | ) | |||||||||||||||||||||||||||||||||||||||||
| Balance, December 31, 2022 | $ | $ | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | $ | ( | ) | $ | - | $ | ||||||||||||||||||||||||||||||
See accompanying notes to the consolidated financial statements.
F-5
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Adjustments to reconcile net loss to | ||||||||
| net cash used in operating activities: | ||||||||
| Bad debt provision | ||||||||
| Depreciation | ||||||||
| Change in straight-line rent receivable | ( | ) | ( | ) | ||||
| Amortization of right-of-use asset | ||||||||
| Stock-based compensation and service expense | ||||||||
| Loss on equity method investment | ||||||||
| Loss on impairment of equipment held for sale | ||||||||
| Amortization of debt discount | ||||||||
| Amortization of debt issuance costs | ||||||||
| Conversion inducement expense | ||||||||
| Change in fair market value of derivative liability | ( | ) | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Rent receivable | ( | ) | ( | ) | ||||
| Rent receivable - related party | ( | ) | ( | ) | ||||
| Security deposit | ( | ) | ||||||
| Deferred leasing costs | ||||||||
| Other assets | ( | ) | ||||||
| Accrued liabilities and other payables | ||||||||
| Accrued liabilities and other payables - related parties | ||||||||
| Operating lease obligation | ( | ) | ( | ) | ||||
| NET CASH USED IN OPERATING ACTIVITIES | ( | ) | ( | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Purchase of property and equipment | ( | ) | ( | ) | ||||
| Improvement of commercial real estate | ( | ) | ||||||
| Additional investment in equity method investment | ( | ) | ( | ) | ||||
| Payments for equity interest purchase | ( | ) | ||||||
| NET CASH USED IN INVESTING ACTIVITIES | ( | ) | ( | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Repayments of note payable - related party | ( | ) | ||||||
| Proceeds from loan payable - related party | ||||||||
| Repayments of loan payable - related party | ( | ) | ||||||
| Proceeds from issuance of convertible debt and warrants | ||||||||
| Proceeds from issuance of balloon promissory note | ||||||||
| Payments of debt issuance costs | ( | ) | ||||||
| Proceeds from equity offering | ||||||||
| Disbursements for equity offering costs | ( | ) | ( | ) | ||||
| Proceeds from issuance of convertible preferred stock | ||||||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES | ||||||||
| EFFECT OF EXCHANGE RATE ON CASH | ||||||||
| NET INCREASE IN CASH | ||||||||
| CASH - beginning of year | ||||||||
| CASH - end of year | $ | $ | ||||||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | ||||||||
| Cash paid for: | ||||||||
| Interest | $ | $ | ||||||
| NON-CASH INVESTING AND FINANCING ACTIVITIES: | ||||||||
| Common stock issued for future services | $ | $ | ||||||
| Common stock issued for accrued liabilities | $ | $ | ||||||
| Deferred financing costs in accrued liabilities | $ | $ | ||||||
| Accrued professional fees relieved for shares issued | $ | $ | ||||||
| Warrants issued with convertible note payable recorded as debt discount | $ | $ | ||||||
| Bifurcated embedded conversion feature recorded as derivative liability and debt discount | $ | $ | ||||||
| Conversion of convertible note payable and accrued interest into common stock | $ | $ | ||||||
| Reclassification of derivative liability to equity | $ | $ | ||||||
| Related party loan and accrued interest settled in shares | $ | $ | ||||||
See accompanying notes to the consolidated financial statements.
F-6
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – ORGANIZATION AND NATURE OF OPERATIONS
Avalon GloboCare Corp. (the “Company”
or “ALBT”) is a Delaware corporation. The Company was incorporated under the laws of the State of Delaware on July 28, 2014.
On October 19, 2016, the Company entered into and closed a Share Exchange Agreement with the shareholders of Avalon Healthcare System,
Inc., a Delaware corporation (“AHS”), each of which were accredited investors (“AHS Shareholders”) pursuant to
which we acquired
For accounting purposes, AHS was the surviving
entity. The transaction was accounted for as a recapitalization of AHS pursuant to which AHS was treated as the accounting acquirer, surviving
and continuing entity although the Company is the legal acquirer. The Company did not recognize goodwill or any intangible assets in connection
with this transaction. Accordingly, the Company’s historical financial statements are those of AHS and its wholly-owned subsidiary,
Avalon (Shanghai) Healthcare Technology Co., Ltd. (“Avalon Shanghai”) immediately following the consummation of this reverse
merger transaction. AHS owns
The Company is a clinical-stage biotechnology company dedicated to developing and delivering innovative, transformative cellular therapeutics, precision diagnostics, and clinical laboratory services. The Company also provides strategic advisory and outsourcing services to facilitate and enhance its clients’ growth and development, as well as competitiveness in healthcare and CellTech industry markets. Through its subsidiary structure with unique integration of verticals from innovative research and development to automated bioproduction and accelerated clinical development, the Company is establishing a leading role in the fields of cellular immunotherapy (including CAR-T/NK), exosome technology (ACTEX™), and regenerative therapeutics.
On January 23, 2017, the Company incorporated Avalon (BVI) Ltd., a British Virgin Island company. There was no activity for the subsidiary since its incorporation through December 31, 2022. Avalon (BVI) Ltd. is dormant and is in process of being dissolved.
On February 7, 2017, the Company formed Avalon
RT 9 Properties, LLC (“Avalon RT 9”), a New Jersey limited liability company. On May 5, 2017, Avalon RT 9 purchased a real
property located in Township of Freehold, County of Monmouth, State of New Jersey, having a street address of 4400 Route 9 South, Freehold,
NJ 07728. This property was purchased to serve as the Company’s world-wide headquarters for all corporate administration and operations.
In addition, the property generates rental income. Avalon RT 9 owns this office building. Avalon RT 9’s business consists of the
ownership and operation of the income-producing real estate property in New Jersey. As of December 31, 2022, the occupancy rate of the
building is
On July 18, 2018, the Company formed a wholly
owned subsidiary, Avactis Biosciences Inc. (“Avactis”), a Nevada corporation, which will focus on accelerating commercial
activities related to cellular therapies, including regenerative medicine with stem/progenitor cells as well as cellular immunotherapy
including CAR-T, CAR-NK, TCR-T and others. The subsidiary is designed to integrate and optimize our global scientific and clinical resources
to further advance the use of cellular therapies to treat certain cancers. Commencing on April 6, 2022, the Company owns
In order to purchase a membership interest, on October 14, 2022, the Company formed a wholly owned subsidiary, Avalon Laboratory Services, Inc., a Delaware company.
F-7
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – ORGANIZATION AND NATURE OF OPERATIONS (continued)
Details of the Company’s subsidiaries which are included in these consolidated financial statements as of December 31, 2022 are as follows:
| Name of Subsidiary | Place and date of Incorporation | Percentage of Ownership | Principal Activities | |||
|
Avalon Healthcare System, Inc. (“AHS”) |
Delaware May 18, 2015 |
|||||
|
Avalon (BVI) Ltd. (“Avalon BVI”) |
British Virgin Island January 23, 2017 |
Dormant, is in process of being dissolved | ||||
|
Avalon RT 9 Properties LLC (“Avalon RT 9”) |
New Jersey February 7, 2017 |
|||||
|
Avalon (Shanghai) Healthcare Technology Co., Ltd. (“Avalon Shanghai”) |
PRC April 29, 2016 |
|||||
|
Genexosome Technologies Inc. (“Genexosome”) |
Nevada July 31, 2017 |
|||||
|
Avactis Biosciences Inc. (“Avactis”) |
Nevada July 18, 2018 |
|||||
|
Avactis Nanjing Biosciences Ltd. (“Avactis Nanjing”) |
PRC May 8, 2020 |
|||||
|
International Exosome Association LLC (“Exosome”) |
Delaware June 13, 2019 |
|||||
| Avalon Laboratory Services, Inc. | Delaware October 14, 2022 |
NOTE 2 – BASIS OF PRESENTATION AND GOING CONCERN CONDITION
Basis of Presentation
The accompanying consolidated financial statements and related notes have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and with the rules and regulations of the U.S. Securities and Exchange Commission for financial information.
The Company’s consolidated financial statements include the accounts of the Company and its subsidiaries. All significant intercompany accounts and transactions have been eliminated in consolidation.
F-8
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2 – BASIS OF PRESENTATION AND GOING CONCERN CONDITION (continued)
Going Concern
The Company is a clinical-stage biotechnology company dedicated to developing and delivering innovative, transformative cellular therapeutics, precision diagnostics, and clinical laboratory services. The Company also provides strategic advisory and outsourcing services to facilitate and enhance its clients’ growth and development, as well as competitiveness in healthcare and CellTech industry markets. Through its subsidiary structure with unique integration of verticals from innovative research and development to automated bioproduction and accelerated clinical development, the Company is establishing a leading role in the fields of cellular immunotherapy (including CAR-T/NK), exosome technology (ACTEX™), and regenerative therapeutics.
In addition, the Company owns commercial real estate that houses its headquarters in Freehold, New Jersey. These consolidated financial statements have been prepared assuming that the Company will continue as a going concern, which contemplates, among other things, the realization of assets and the satisfaction of liabilities in the normal course of business.
As reflected in the accompanying consolidated financial statements,
the Company had working capital deficit of $
The occurrence of an uncontrollable event such as the COVID-19 pandemic had negatively impact on the Company’s operations. Our general development operations have continued during the COVID-19 pandemic and we have not had significant disruption. However, we are uncertain if the COVID-19 pandemic will impact future operations at our laboratory, or our ability to collaborate with other laboratories and universities. In addition, we are unsure if the COVID-19 pandemic will impact future clinical trials. Given the dynamic nature of these circumstances, the duration of business disruption and reduced traffic, the related financial effect cannot be reasonably estimated at this time.
The accompanying consolidated financial statements do not include any adjustments related to the recoverability or classification of asset-carrying amounts or the amounts and classification of liabilities that may result should the Company be unable to continue as a going concern.
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Use of Estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Changes in these estimates and assumptions may have a material impact on the consolidated financial statements and accompanying notes. Making estimates requires management to exercise significant judgment. It is at least reasonably possible that the estimate of the effect of a condition, situation or set of circumstances that existed at the date of the financial statements, which management considered in formulating its estimate, could change in the near term due to one or more future confirming events. Accordingly, the actual results could differ significantly from those estimates. Significant estimates during the years ended December 31, 2022 and 2021 include the useful life of property and equipment and investment in real estate, assumptions used in assessing impairment of long-term assets, valuation of deferred tax assets and the associated valuation allowances, valuation of stock-based compensation, and assumptions used to determine fair value of warrants and embedded conversion features of convertible note payable.
F-9
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Fair Value of Financial Instruments and Fair Value Measurements
The Company adopted the guidance of Accounting Standards Codification (“ASC”) 820 for fair value measurements which clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
| ● | Level 1-Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date. |
| ● | Level 2-Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other than quoted prices that are observable, and inputs derived from or corroborated by observable market data. |
| ● | Level 3-Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information. |
The fair value of the Company’s assets and liabilities, which qualify as financial instruments under ASC Topic 820, “Fair Value Measurement,” approximates the carrying amounts represented in the accompanying consolidated financial statements, primarily due to their short-term nature.
Assets and liabilities measured at fair value on a recurring basis. Certain assets and liabilities are measured at fair value on a recurring basis. These assets and liabilities are measured at fair value on an ongoing basis. These assets and liabilities include derivative liability.
Derivative liability. Derivative liability is carried at fair value and measured on an ongoing basis. The Company did not have any derivative liability during the year ended December 31, 2021. The table below reflects the activity of derivative liability measured at fair value for the year ended December 31, 2022:
| Significant Unobservable Inputs (Level 3) | ||||
| Balance of derivative liability as of January 1, 2022 | $ | |||
| Initial fair value of derivative liability attributable to embedded conversion feature of convertible note payable | ||||
| Gain from change in the fair value of derivative liability | ( | ) | ||
| Reclassification of derivative liability to equity | ( | ) | ||
| Balance of derivative liability as of December 31, 2022 | $ | |||
ASC 825-10 “Financial Instruments”, allows entities to voluntarily choose to measure certain financial assets and liabilities at fair value (fair value option). The fair value option may be elected on an instrument-by-instrument basis and is irrevocable, unless a new election date occurs. If the fair value option is elected for an instrument, unrealized gains and losses for that instrument should be reported in earnings at each subsequent reporting date. The Company did not elect to apply the fair value option to any outstanding instruments.
Cash and Cash Equivalents
At December 31, 2022 and 2021, the Company’s cash balances by geographic area were as follows:
| Country: | December 31, 2022 | December 31, 2021 | ||||||||||||||
| United States | $ | % | $ | % | ||||||||||||
| China | % | % | ||||||||||||||
| Total cash | $ | % | $ | % | ||||||||||||
For purposes of the consolidated statements of cash flows, the Company considers all highly liquid instruments with a maturity of three months or less when purchased and money market accounts to be cash equivalents. The Company had no cash equivalents at December 31, 2022 and 2021.
F-10
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Advances for Equity Interest Purchase
In the fourth quarter of 2022, the Company sold
Credit Risk and Uncertainties
A portion
of the Company’s cash is maintained with state-owned banks within the PRC.
The Company
maintains a portion of its cash in bank and financial institution deposits within U.S. that at times may exceed federally-insured limits
of $
Financial instruments which potentially subject the Company to concentrations of credit risk consist principally of trade accounts receivable. A portion of the Company’s sales are credit sales which is to the customer whose ability to pay is dependent upon the industry economics prevailing in these areas; however, concentrations of credit risk with respect to trade accounts receivable is limited due to short-term payment terms. The Company also performs ongoing credit evaluations of its customers to help further reduce credit risk.
Rent Receivable and Allowance for Doubtful Accounts
Rent receivable is presented net of an allowance for doubtful accounts. Rent receivable balance consists of base rents, tenant reimbursements and receivables arising from straight-lining of rents represent amounts accrued and unpaid from tenants in accordance with the terms of the respective leases, subject to the Company’s revenue recognition policy. An allowance for the uncollectible portion of rent receivable is determined based upon an analysis of the tenant’s payment history, the financial condition of the tenant, business conditions in the industry in which the tenant operates and economic conditions in Freehold, New Jersey in which the property is located.
Management believes that the rent receivable is fully collectable. Therefore, no material allowance for doubtful accounts is deemed to be required on its rent receivable at December 31, 2022 and 2021.
Deferred financing costs
Deferred
financing costs consist of legal, accounting and other costs that are directly related to the Company’s open market sale equity
financing and will be charged to stockholders’ equity upon the completion of the equity offering. As of December 31, 2022 and 2021,
deferred financing costs amounted to $
Debt Issuance Costs
Debt issuance costs are
those costs that have been incurred in connection with the issuance of balloon promissory note payable in 2022 and are offset against
note payable in the consolidated balance sheets. Such costs are being amortized to interest expense over the term of the underlying debt
using the straight-line method, as the difference between that and the effective interest method are immaterial. As
of December 31, 2022, debt issuance costs amounted to $
F-11
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Deferred leasing costs
Costs incurred to obtain tenant leases are amortized using the straight-line method over the term of the related lease agreement. Such costs include lease incentives and leasing commissions. If the lease is terminated early, the remaining unamortized deferred leasing cost is written off.
Property and Equipment
Property and equipment are carried at cost and are depreciated on a straight-line basis over the estimated useful lives of the assets. The cost of repairs and maintenance is expensed as incurred; major replacements and improvements are capitalized. When assets are retired or disposed of, the cost and accumulated depreciation are removed from the accounts, and any resulting gains or losses are included in income in the period of disposition. The Company examines the possibility of decreases in the value of fixed assets when events or changes in circumstances reflect the fact that their recorded value may not be recoverable.
Investment In Real Estate and Depreciation
Investment in real estate is carried at cost less accumulated depreciation and consists of building and improvement. The Company depreciates real estate building and improvement on a straight-line basis over estimated useful life. Expenditures for ordinary repair and maintenance costs are charged to expense as incurred. Expenditure for improvements, renovations, and replacements of real estate asset is capitalized and depreciated over its estimated useful life if the expenditure qualifies as betterment.
Impairment of Long-lived Assets
In accordance with ASC Topic 360, the Company reviews long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable, or at least annually. The Company recognizes an impairment loss when the sum of expected undiscounted future cash flows is less than the carrying amount of the asset. The amount of impairment is measured as the difference between the asset’s estimated fair value and its book value. The Company did not record any impairment charge for the years ended December 31, 2022 and 2021.
Investment in Unconsolidated Company – Epicon Biosciences Co., Ltd.
The Company uses the equity method of accounting for its investment in, and earning or loss of, company that it does not control but over
which it does exert significant influence. The Company considers whether the fair value of its equity method investment has declined below
its carrying value whenever adverse events or changes in circumstances indicate that recorded value may not be recoverable. If the Company
considers any decline to be other than temporary (based on various factors, including historical financial results and the overall health
of the investee), then a write-down would be recorded to estimated fair value. See Note 7 for discussion of equity method investment.
Deferred Rental Income
Deferred
rental income represents rental income collected but not earned as of the reporting date. The Company defers the revenue related to lease
payments received from tenants in advance of their due dates. As of December 31, 2022 and 2021, deferred rental income totaled $
F-12
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Revenue Recognition
The Company recognizes revenue under Accounting Standards Codification (“ASC”) Topic 606, Revenue from Contracts with Customers (“ASC 606”). The core principle of the revenue standard is that a company should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. The following five steps are applied to achieve that core principle:
| ● | Step 1: Identify the contract with the customer |
| ● | Step 2: Identify the performance obligations in the contract |
| ● | Step 3: Determine the transaction price |
| ● | Step 4: Allocate the transaction price to the performance obligations in the contract |
| ● | Step 5: Recognize revenue when the company satisfies a performance obligation |
In order to identify the performance obligations in a contract with a customer, a company must assess the promised goods or services in the contract and identify each promised goods or service that is distinct. A performance obligation meets ASC 606’s definition of a “distinct” goods or service (or bundle of goods or services) if both of the following criteria are met:
| ● | The customer can benefit from the goods or service either on its own or together with other resources that are readily available to the customer (i.e., the goods or service is capable of being distinct). |
| ● | The entity’s promise to transfer the goods or service to the customer is separately identifiable from other promises in the contract (i.e., the promise to transfer the goods or service is distinct within the context of the contract). |
If a goods or service is not distinct, the goods or service is combined with other promised goods or services until a bundle of goods or services is identified that is distinct.
The transaction price is the amount of consideration to which an entity expects to be entitled in exchange for transferring promised goods or services to a customer, excluding amounts collected on behalf of third parties (for example, some sales taxes). The consideration promised in a contract with a customer may include fixed amounts, variable amounts, or both. Variable consideration is included in the transaction price only to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved.
The transaction price is allocated to each performance obligation on a relative standalone selling price basis. The transaction price allocated to each performance obligation is recognized when that performance obligation is satisfied, at a point in time or over time as appropriate.
The Company’s revenues are derived from providing medial related consulting services for its’ related parties. Revenues related to its service offerings are recognized at a point in time when service is rendered. Any payments received in advance of the performance of services are recorded as deferred revenue until such time as the services are performed.
The Company has determined that the ASC 606 does not apply to rental contracts, which are within the scope of other revenue recognition accounting standards.
Rental income from operating leases is recognized on a straight-line basis under the guidance of ASC 842. Lease payments under tenant leases are recognized on a straight-line basis over the term of the related leases. The cumulative difference between lease revenue recognized under the straight-line method and contractual lease payments are included in rent receivable on the consolidated balance sheets.
The Company does not offer promotional payments, customer coupons, rebates or other cash redemption offers to its customers.
F-13
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Office Lease
When a lease contains “rent holidays”, the Company records rental expense on a straight-line basis over the term of the lease. The Company begins recording rent expense on the lease possession date.
Real Property Operating Expenses
Real property operating expenses consist of property management fees, property insurance, real estate taxes, depreciation, repairs and maintenance fees, utilities and other expenses related to the Company’s rental properties.
Medical Related Consulting Services Costs
Costs of medical related consulting services include the cost of labor and related benefits, travel expenses related to consulting services, and other overhead costs.
Research and Development
Expenditures
for research and product development costs are expensed as incurred. The Company incurred research and development expense of $
Advertising and Marketing Costs
All costs
related to advertising and marketing are expensed as incurred. For the years ended December 31, 2022 and 2021, advertising and marketing
costs amounted to $
Stock-based Compensation
The Company accounts for its stock-based compensation awards in accordance with Accounting Standards Codification (“ASC”) Topic 718, Compensation—Stock Compensation (“ASC 718”). ASC 718 requires all stock-based payments to employees and non-employees including grants of stock options, to be recognized as expense in the statements of operations based on their grant date fair values. The Company estimates the grant date fair value of each option award using the Black-Scholes option-pricing model.
The Company periodically issues common stock and common stock options to consultants for various services. Costs of these transactions are measured at the fair value of the service received or the fair value of the equity instruments issued, whichever is more reliably measurable. The value of the common stock is measured at the earlier of (i) the date at which a firm commitment for performance by the counterparty to earn the equity instruments is reached or (ii) the date at which the counterparty’s performance is complete.
Income Taxes
The Company is governed by the income tax laws of China and the United States. The Company accounts for income taxes using the asset/liability method prescribed by ASC 740, “Income Taxes.” Under this method, deferred tax assets and liabilities are determined based on the difference between the financial reporting and tax bases of assets and liabilities using enacted tax rates that will be in effect in the period in which the differences are expected to reverse. The Company records a valuation allowance to offset deferred tax assets if, based on the weight of available evidence, it is more-likely-than-not that some portion, or all, of the deferred tax assets will not be realized. The effect on deferred taxes of a change in tax rates is recognized as income or loss in the period that includes the enactment date.
The Company follows the accounting guidance for uncertainty in income taxes using the provisions of ASC 740 “Income Taxes”. Using that guidance, the benefit for tax positions taken can only be recognized in the financial statements when it is more likely than not the position will be sustained upon examination by the tax authorities. As of December 31, 2022 and 2021, the Company had no significant uncertain tax positions which would require either recognition of a liability or disclosure in the financial statements. For United States entities, tax year that remains subject to examination is the years ended December 31, 2022, 2021, 2020 and 2019. For China entities, income tax returns for the tax years ended December 31, 2018 through December 31, 2022 remain open for statutory examination by PRC tax authorities. The Company recognizes interest and penalties related to significant uncertain income tax positions in income tax expense. However, no such interest and penalties were recorded as of December 31, 2022 and 2021.
F-14
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Foreign Currency Translation
The reporting currency of the Company is the U.S. dollar. The functional currency of the parent company, AHS, Avalon RT 9, Genexosome, Avactis, and Exosome, is the U.S. dollar and the functional currency of Avalon Shanghai is the Chinese Renminbi (“RMB”). For the subsidiaries whose functional currency is the RMB, result of operations and cash flows are translated at average exchange rates during the period, assets and liabilities are translated at the unified exchange rate at the end of the period, and equity is translated at historical exchange rates. As a result, amounts relating to assets and liabilities reported on the statements of cash flows may not necessarily agree with the changes in the corresponding balances on the balance sheets. Translation adjustments resulting from the process of translating the local currency financial statements into U.S. dollars are included in determining comprehensive income/loss. Transactions denominated in foreign currencies are translated into the functional currency at the exchange rates prevailing on the transaction dates. Assets and liabilities denominated in foreign currencies are translated into the functional currency at the exchange rates prevailing at the balance sheet date with any transaction gains and losses that arise from exchange rate fluctuations on transactions denominated in a currency other than the functional currency are included in the results of operations as incurred. All of the Company’s revenue transactions are transacted in the functional currency of the operating subsidiaries. The Company does not enter into any material transaction in foreign currencies. Transaction gains or losses have not had, and are not expected to have, a material effect on the results of operations of the Company.
Comprehensive Loss
Comprehensive loss is comprised of net loss and all changes to the statements of equity, except those due to investments by stockholders, changes in paid-in capital and distributions to stockholders. For the Company, comprehensive loss for the years ended December 31, 2022 and 2021 consisted of net loss and unrealized (loss) gain from foreign currency translation adjustment.
Per Share Data
ASC Topic 260 “Earnings per Share,” requires presentation of both basic and diluted earnings per share (“EPS”) with a reconciliation of the numerator and denominator of the basic EPS computation to the numerator and denominator of the diluted EPS computation. Basic EPS excludes dilution. Diluted EPS reflects the potential dilution that could occur if securities or other contracts to issue common stock were exercised or converted into common stock or resulted in the issuance of common stock that then shared in the earnings of the entity.
Basic net loss per share is computed by dividing net loss available to common stockholders by the weighted average number of shares of common stock outstanding during the period. Diluted net loss per share is computed by dividing net loss by the weighted average number of shares of common stock, common stock equivalents and potentially dilutive securities outstanding during each period. For the years ended December 31, 2022 and 2021, potentially dilutive common shares consist of the common shares issuable upon the conversion of Series A convertible preferred stock (using the if-converted method) and exercise of common stock options and warrants (using the treasury stock method). Common stock equivalents are not included in the calculation of diluted net loss per share if their effect would be anti-dilutive. In a period in which the Company has a net loss, all potentially dilutive securities are excluded from the computation of diluted shares outstanding as they would have had an anti-dilutive impact.
F-15
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Per Share Data (continued)
The following table summarizes the securities that were excluded from the diluted per share calculation because the effect of including these potential shares was antidilutive:
| Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Options to purchase common stock | ||||||||
| Warrants to purchase common stock | ||||||||
| Convertible note (*) | ||||||||
| Series A convertible preferred stock (**) | ||||||||
| Potentially dilutive securities | ||||||||
| (*) |
| (**) |
Non-controlling Interest
As of December 31, 2022, Dr. Yu Zhou, former director
and former Co-Chief Executive Officer of Genexosome, who owns
Segment Reporting
The Company uses “the management approach” in determining reportable operating segments. The management approach considers the internal organization and reporting used by the Company’s chief operating decision maker for making operating decisions and assessing performance as the source for determining the Company’s reportable segments. The Company’s chief operating decision maker is the Chief Executive Officer (“CEO”) and president of the Company, who reviews operating results to make decisions about allocating resources and assessing performance for the entire Company.
During the years ended December 31, 2022 and 2021, the Company operated in two reportable business segments - (1) the real property operating segment, and (2) the medical related consulting services segment. These reportable segments offer different services and products, have different types of revenue, and are managed separately as each requires different operating strategies and management expertise. Due to the winding down of the medical related consulting services segment in 2022, the Company decided to cease all operations of this segment and no longer has any material revenues or expenses in this segment. As a result, commencing from the first quarter of 2023, the Company’s chief operating decision maker no longer reviews medical related consulting services operating results.
F-16
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Related Parties
Parties are considered to be related to the Company if the parties, directly or indirectly, through one or more intermediaries, control, are controlled by, or are under common control with the Company. Related parties also include principal owners of the Company, its management, members of the immediate families of principal owners of the Company and its management and other parties with which the Company may deal with if one party controls or can significantly influence the management or operating policies of the other to an extent that one of the transacting parties might be prevented from fully pursuing its own separate interests. The Company discloses all significant related party transactions.
Reclassification
Certain prior period amounts have been reclassified to conform to the current period presentation. These reclassifications have no effect on the previously reported financial position, results of operations and cash flows.
Fiscal Year End
The Company has adopted a fiscal year end of December 31st.
Reverse Stock Split
The Company effected a one-for-ten reverse stock split of its outstanding shares of common stock on January 5, 2023. The reverse split did not change the number of authorized shares of common stock or par value. All references in these consolidated financial statements to shares, share prices, exercise prices, and other per share information in all periods have been adjusted, on a retroactive basis, to reflect the reverse stock split.
Recent Accounting Standards
In August 2020, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2020-06, Debt - Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging - Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (“ASU 2020-06”), which simplifies the accounting for certain financial instruments with characteristics of liabilities and equity. This ASU (1) simplifies the accounting for convertible debt instruments and convertible preferred stock by removing the existing guidance in ASC 470-20, Debt: Debt with Conversion and Other Options, that requires entities to account for beneficial conversion features and cash conversion features in equity, separately from the host convertible debt or preferred stock; (2) revises the scope exception from derivative accounting in ASC 815-40 for freestanding financial instruments and embedded features that are both indexed to the issuer’s own stock and classified in stockholders’ equity, by removing certain criteria required for equity classification; and (3) revises the guidance in ASC 260, Earnings Per Share, to require entities to calculate diluted earnings per share (EPS) for convertible instruments by using the if-converted method. In addition, entities must presume share settlement for purposes of calculating diluted EPS when an instrument may be settled in cash or shares. ASU 2020-06 is effective for public business entities for fiscal years beginning after December 15, 2021 (or December 15, 2023 for companies who meet the SEC definition of Smaller Reporting Companies), and interim periods within those fiscal years. The guidance is to be adopted through either a fully retrospective or modified retrospective method of transition. However, early adoption is permitted as early as fiscal years, and interim periods within those fiscal years, beginning after December 15, 2020. The Company adopted the new standard on January 1, 2022, which adoption required the Company to bifurcate the embedded conversion feature from the convertible note it issued during the second quarter of 2022.
In June 2016, the FASB issued ASU 2016-13, Financial Instruments - Credit Losses (“Topic 326”). The ASU introduces a new accounting model, the Current Expected Credit Losses model (“CECL”), which requires earlier recognition of credit losses and additional disclosures related to credit risk. The CECL model utilizes a lifetime expected credit loss measurement objective for the recognition of credit losses at the time the financial asset is originated or acquired. ASU 2016-13 is effective for annual period beginning after December 15, 2022, including interim reporting periods within those annual reporting periods. The Company expects that the adoption will not have a material impact on the Company’s consolidated financial statements.
Other accounting standards that have been issued or proposed by FASB that do not require adoption until a future date are not expected to have a material impact on the consolidated financial statements upon adoption. The Company does not discuss recent pronouncements that are not anticipated to have an impact on or are unrelated to its consolidated financial condition, results of operations, cash flows or disclosures.
F-17
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 4 – OTHER CURRENT AND NON-CURRENT ASSETS
At December 31, 2022 and 2021, other current and non-current assets consisted of the following:
| December 31, 2022 | December 31, 2021 | |||||||
| Prepaid directors and officers liability insurance premium | $ | $ | ||||||
| Prepaid professional fees | ||||||||
| Deferred financing costs, net | ||||||||
| Recoverable VAT | ||||||||
| Deferred leasing costs | ||||||||
| Security deposit | ||||||||
| Equipment held for sale | ||||||||
| Long-term straight-line rent receivable | ||||||||
| Others | ||||||||
| Total | $ | $ | ||||||
| Current portion | $ | $ | ||||||
| Non-current portion | ||||||||
| Total | $ | $ | ||||||
NOTE 5 – PROPERTY AND EQUIPMENT
At December 31, 2022 and 2021, property and equipment consisted of the following:
| Useful life | December 31, 2022 | December 31, 2021 | ||||||||
| Laboratory equipment | $ | $ | ||||||||
| Office equipment and furniture | ||||||||||
| Less: accumulated depreciation | ( | ) | ( | ) | ||||||
| $ | $ | |||||||||
For the years ended December
31, 2022 and 2021, depreciation expense of property and equipment amounted to $
NOTE 6 – INVESTMENT IN REAL ESTATE
At December 31, 2022 and 2021, investment in real estate consisted of the following:
| Useful life | December 31, 2022 | December 31, 2021 | ||||||||
| Commercial real property building | $ | $ | ||||||||
| Improvement | ||||||||||
| Less: accumulated depreciation | ( | ) | ( | ) | ||||||
| $ | $ | |||||||||
For the years ended December
31, 2022 and 2021, depreciation expense of this commercial real property amounted to $
F-18
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 7 – EQUITY METHOD INVESTMENT
As of December
31, 2022 and 2021, the equity method investment amounted to $
The Company treats the equity investment in the consolidated financial statements under the equity method. Under the equity method, the investment is initially recorded at cost, adjusted for any excess of the Company’s share of the incorporated-date fair values of the investee’s identifiable net assets over the cost of the investment (if any). Thereafter, the investment is adjusted for the post incorporation change in the Company’s share of the investee’s net assets and any impairment loss relating to the investment.
For the
years ended December 31, 2022 and 2021, the Company’s share of Epicon’s net loss was $
In the years ended December 31, 2022 and 2021, activity recorded for the Company’s equity method investment in Epicon is summarized in the following table:
| Equity investment carrying amount at January 1, 2021 | $ | |||
| Payment made for equity method investment | ||||
| Epicon’s net loss attributable to the Company | ( | ) | ||
| Foreign currency fluctuation | ||||
| Equity investment carrying amount at December 31, 2021 | ||||
| Payment made for equity method investment | ||||
| Epicon’s net loss attributable to the Company | ( | ) | ||
| Foreign currency fluctuation | ( | ) | ||
| Equity investment carrying amount at December 31, 2022 | $ |
The tables below present the summarized financial information, as provided to the Company by the investee, for the unconsolidated company:
| December 31, 2022 | December 31, 2021 | |||||||
| Current assets | $ | $ | ||||||
| Noncurrent assets | ||||||||
| Current liabilities | ||||||||
| Equity | ||||||||
| For the Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Net revenue | $ | $ | ||||||
| Gross profit | ||||||||
| Loss from operation | ||||||||
| Net loss | ||||||||
F-19
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 8 – ACCRUED LIABILITIES AND OTHER PAYABLES
At December 31, 2022 and 2021, accrued liabilities and other payables consisted of the following:
| December 31, 2022 | December 31, 2021 | |||||||
| Accrued tenants’ improvement reimbursement | $ | $ | ||||||
| Tenants’ security deposit | ||||||||
| Accrued business expense reimbursement | ||||||||
| Accrued utilities | ||||||||
| Deferred rental income | ||||||||
| Accrued real property cleaning service fee | ||||||||
| Accrued equity offering costs | ||||||||
| Taxes payable | ||||||||
| Others | ||||||||
| Total | $ | $ | ||||||
NOTE 9 – CONVERTIBLE NOTE PAYABLE
On March 28, 2022, the
Company entered into Securities Purchase Agreement with an accredited investor, which was amended on June 8, 2022, providing for the sale
by the Company to the investor of a Convertible Note in the amount of $
| ● | $ |
| ● | $ |
| ● | $ |
| ● | $ |
As
a result of each of the closings, the Company issued the investor a 2022 Convertible Note in the principal amount of $
The 2022
Convertible Note bears interest at
The investor
agreed to restrict its ability to convert the 2022 Convertible Note and exercise the 2022 Warrant and receive shares of common stock such
that the number of shares of common stock held by the investor after such conversion or exercise does not exceed
F-20
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 9 – CONVERTIBLE NOTE PAYABLE (continued)
Based upon the Company’s analysis of the criteria contained in ASC Topic 815-40, “Derivatives and Hedging - Contracts in an Entity’s Own Equity”, the Company determined that all the warrants issued to the investor with this private placement are classified as equity in additional paid in-capital.
In accordance with ASC 470-20-25-2, proceeds from the sale of a debt instrument with stock purchase warrants are allocated to the two elements based on the relative fair values of the debt instrument without the warrants and of the warrants themselves at time of issuance. The portion of the proceeds so allocated to the warrants are accounted for as additional paid-in capital. The remainder of the proceeds are allocated to the debt instrument portion of the transaction.
The fair
values of the warrants issued to the investor with this private placement were computed using the Black-Scholes option-pricing model with
the following assumptions: volatility of
In accordance
with ASC 480-10-25-14, the Company determined that the conversion provisions contain an embedded derivative feature and the Company valued
the derivative feature separately, recording debt discount and derivative liabilities in accordance with the provisions of the convertible
debt (see Note 10). The Company calculates the fair value of conversion option at the commitment dates using the Black-Scholes valuation
model with the following assumptions: volatility of
The warrants
issued to the investor to purchase
On July 25, 2022, the Company and the investor
entered into a Conversion Agreement (“Conversion Agreement”) pursuant to which the investor converted all of its Convertible
Notes in the principal amount of $
For the
year ended December 31, 2022, amortization of debt discount and interest expense related to the 2022 Convertible Note amounted to $
NOTE 10 – DERIVATIVE LIABILITY
As stated in Note 9, 2022 Convertible Note, the Company determined that the convertible note payable contained an embedded derivative feature in the form of a conversion provision which was adjustable based on future prices of the Company’s common stock. In accordance with ASC 815-10-25, each derivative feature was initially recorded at its fair value using the Black-Scholes option valuation method and then re-valued at each reporting date, with changes in the fair value reported in the statements of operations.
The estimated
fair value of the derivative feature of convertible debt was $
F-21
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 10 – DERIVATIVE LIABILITY (continued)
Increases or decreases in fair
value of the derivative liability is included as a component of total other (expenses) income in the accompanying consolidated statements
of operations and comprehensive loss. The change to the derivative liability for the embedded conversion option resulted in a decrease
of $
NOTE 11 – NOTE PAYABLE, NET
On September 1, 2022,
the Company issued a balloon promissory note to a third party company in the principal amount of $
As of December 31, 2022,
the carrying balance of the 2022 Note Payable was $
For the
year ended December 31, 2022, amortization of debt issuance costs and interest expense related to the 2022 Note Payable amounted to $
NOTE 12 – RELATED PARTY TRANSACTIONS
Rental Revenue from Related Party and Rent Receivable – Related Party
The Company leases space of its commercial real property located in New Jersey to a company, D.P. Capital Investments LLC, which is controlled by Wenzhao Lu, the Company’s largest shareholder and chairman of the Board of Directors. The term of the related party lease agreement is five years commencing on May 1, 2021 and will expire on April 30, 2026.
For the years ended December 31, 2022 and 2021,
the related party rental revenue amounted to $
The related party rent receivable totaled $
Medical Related Consulting Services Revenue from Related Party
During the years ended December 31, 2022 and 2021, medical related consulting services revenue from related party was as follows:
| Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Medical related consulting services provided to: | ||||||||
| Hebei Daopei * | $ | $ | ||||||
| $ | $ | |||||||
| * |
Services Provided by Related Party
From time to time, Wilbert Tauzin, a director
of the Company, and his son provide consulting services to the Company. As compensation for professional services provided, the Company
recognized consulting expenses of $
F-22
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 12 – RELATED PARTY TRANSACTIONS (continued)
Accrued Liabilities and Other Payables – Related Parties
In 2017,
the Company acquired Beijing Genexosome for a cash payment of $
As of December
31, 2022 and 2021, $
Borrowings from Related Party
Promissory Note
On March 18, 2019, the
Company issued Wenzhao Lu, the Company’s largest shareholder and Chairman of the Board of Directors, a Promissory Note in the principal
amount of $
Line of Credit
On August
29, 2019, the Company entered into a Line of Credit Agreement (the “Line of Credit Agreement”) providing the Company with
a $
In the years ended December 31, 2022 and 2021, activity recorded for the Line of Credit is summarized in the following table:
| Outstanding principal under the Line of Credit at January 1, 2021 | $ | |||
| Draw down from Line of Credit | ||||
| Settlement of Line of Credit in shares | ( | ) | ||
| Outstanding principal under the Line of Credit at December 31, 2021 | ||||
| Draw down from Line of Credit | ||||
| Repayment of Line of Credit | ( | ) | ||
| Settlement of Line of Credit in shares | ( | ) | ||
| Outstanding principal under the Line of Credit at December 31, 2022 | $ |
For the
years ended December 31, 2022 and 2021, the interest expense related to above borrowings amounted to $
As of December
31, 2022 and 2021, the related accrued and unpaid interest for above borrowings was $
F-23
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 12 – RELATED PARTY TRANSACTIONS (continued)
Common Shares Sold to Related Party for Cash
On August 5, 2022, the Company sold
Series A Convertible Preferred Stock Sold to Related Party for Cash
On December 14, 2022,
the Company entered into a Securities Purchase Agreement with Wenzhao Lu, the Company’s Chairman of the Board, pursuant to which
the Company sold to Mr. Lu
NOTE 13 – INCOME TAXES
The Company
is governed by the Income Tax Law of the PRC and the U.S. Internal Revenue Code of 1986, as amended. Under the Income Tax Laws of PRC,
Chinese companies are generally subject to an income tax at an effective rate of
The Company’s loss before income taxes includes the following components:
| Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| United States loss before income taxes | $ | ( | ) | $ | ( | ) | ||
| China loss before income taxes | ( | ) | ( | ) | ||||
| Total loss before income taxes | $ | ( | ) | $ | ( | ) | ||
Components of income taxes expense (benefit) consisted of the following:
| Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Current: | ||||||||
| U.S. federal | $ | $ | ||||||
| U.S. state and local | ||||||||
| China | ||||||||
| Total current income taxes expense | $ | $ | ||||||
| Deferred: | ||||||||
| U.S. federal | $ | ( | ) | $ | ( | ) | ||
| U.S. state and local | ( | ) | ( | ) | ||||
| China | ( | ) | ||||||
| Total deferred income taxes (benefit) | $ | ( | ) | $ | ( | ) | ||
| Change in valuation allowance | ||||||||
| Total income taxes expense | $ | $ | ||||||
F-24
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 13 – INCOME TAXES (continued)
The table below summarizes the differences between the U.S. statutory rate and the Company’s effective tax rate for the years ended December 31, 2022 and 2021:
| Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| U.S. federal rate | % | % | ||||||
| U.S. state rate | % | % | ||||||
| Permanent difference | ( | )% | % | |||||
| Non-US rate differential | % | % | ||||||
| True ups | ( | )% | % | |||||
| U.S. valuation allowance | ( | )% | ( | )% | ||||
| Total provision for income taxes | % | % | ||||||
For the years ended December 31, 2022 and 2021, the Company did not incur any income taxes expense since it did not generate any taxable income in those periods. The Company’s foreign entities did not pay any income taxes during the years ended December 31, 2022 and 2021. The Company’s components of deferred taxes as of December 31, 2022 and 2021 were as follows:
| December 31, 2022 | December 31, 2021 | |||||||
| Deferred tax assets | ||||||||
| Stock-based compensation | $ | $ | ||||||
| Disallowed business interest deduction | ||||||||
| R&D expenses | ||||||||
| Accrued directors’ compensation | ||||||||
| Accrued settlement | - | |||||||
| Lease liability | ||||||||
| Net operating loss carryforward | ||||||||
| Total deferred tax assets, gross | ||||||||
| Valuation allowance | ( | ) | ( | ) | ||||
| Total deferred tax assets, net | $ | $ | ||||||
| Deferred tax liabilities | ||||||||
| Fixed assets and intangible assets book/tax basis difference | ( | ) | ( | ) | ||||
| Right-of-use assets | ( | ) | ||||||
| Total deferred tax liabilities | $ | ( | ) | $ | ( | ) | ||
| Net deferred tax assets | $ | $ | ||||||
As of December
31, 2022 and 2021, the Company’s both federal and state net operating loss carryforwards amounted to $
As of December
31, 2022, the Company had net operating loss carryforwards in China of $
Additionally,
as of December 31, 2022, $
A full valuation allowance has been provided against the Company’s deferred tax assets at December 31, 2022 as the Company believes it is more likely than not that sufficient taxable income will not be generated to realize these temporary differences.
The Company
has been notified and assessed an IRS Section 6038 penalty of $
F-25
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 13 – INCOME TAXES (continued)
The Company has not been audited by any jurisdiction since its inception. The Company is open for audit by the U.S. Internal Revenue Service and U.S. state tax jurisdictions from 2019 to 2022, and open for audit by the Chinese Ministry of Finance from 2018 to 2022.
There were no material uncertain tax positions as of December 31, 2022 and 2021. The Company recognizes interest and penalties related to unrecognized tax benefits as income tax expense, if any. The Company does not have any significant uncertain tax positions or events leading to uncertainty in a tax position.
NOTE 14 – EQUITY
Series A Convertible Preferred Stock
As described in Note
20 - Amended and Restated Membership Interest Purchase Agreement, in conjunction with the transaction, on November 3, 2022 the Company
filed a Certificate of Designation of Preferences, Rights and Limitations of the Series A Preferred Stock (the “Series A Certificate
of Designation”), which became effective immediately with the Secretary of State of the State of Delaware. Pursuant to the Series
A Certificate of Designation, the Company designated up to
The shares of Series A Preferred Stock have identical terms and include the terms as set forth below.
Dividends. The Series A Holders are entitled to receive, and the Company shall pay, dividends on shares of Series A Preferred Stock equal (on an as-if-converted-to-common-stock basis, disregarding for such purpose any conversion limitations set forth in the Series A Certificate of Designations) to and in the same form as dividends actually paid on shares of the Company’s common stock when, as and if such dividends are paid on shares of the common stock. No other dividends shall be paid on shares of Series A Preferred Stock. The Company will not pay any dividends on its common stock unless the Company simultaneously complies with the terms set forth in the Series A Certificate of Designation.
Liquidation. Upon any dissolution, liquidation or winding-up of the Company, whether voluntary or involuntary (a “Liquidation”), the Series A Holders will be entitled to receive out of the assets available for distribution to the stockholders, (i) after and subject to the payment in full of all amounts required to be distributed to the holders of another class or series of stock of the Company ranking on liquidation prior and in preference to the Series A Preferred Stock, (ii) ratably with any class or series of stock ranking on liquidation on parity with the Series A Preferred Stock and (iii) in preference and priority to the holders of the shares of the Company’s common stock, an amount equal to 100% of the Series A Stated Value, and no more, in proportion to the full and preferential amount that all shares of the Series A Preferred Stock are entitled to receive. The Company shall mail written notice of any Liquidation not less than twenty (20) days prior to the payment date stated therein, to each Series A Holder.
Conversion. Each
share of Series A Preferred Stock shall be convertible, at any time and from time to time from and after the later of (i) the date of
the stockholder approval as described above, in accordance with the Nasdaq Stock Market Listing Rules, and (ii) the
F-26
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 14 – EQUITY (continued)
Series A Convertible Preferred Stock (continued)
Conversion Price Adjustment:
Stock Dividends and Stock Splits. If the Company, at any time while the Series A Preferred Stock is outstanding: (i) pays a stock dividend or otherwise makes a distribution or distributions payable in shares of common stock on shares of common stock or any other common stock equivalents (which, for avoidance of doubt, shall not include any shares of common stock issued by the Company upon conversion of, or payment of a dividend on, the Series A Preferred Stock), (ii) subdivides outstanding shares of common stock into a larger number of shares, (iii) combines (including by way of a reverse stock split) outstanding shares of common stock into a smaller number of shares, or (iv) issues, in the event of a reclassification of shares of the common stock, any shares of capital stock of the Company, then the conversion price of the Series A Preferred Stock shall be multiplied by a fraction of which the numerator shall be the number of shares of common stock (excluding any treasury shares of the Company) outstanding immediately before such event, and of which the denominator shall be the number of shares of common stock outstanding immediately after such event. Any of the foregoing adjustments shall become effective immediately after the record date for the determination of stockholders entitled to receive such dividend or distribution and shall become effective immediately after the effective date in the case of a subdivision, combination or re-classification.
Fundamental Transaction.
If, at any time while the Series A Preferred Stock is outstanding, (i) the Company, directly or indirectly, in one or more related transactions
effects any merger or consolidation of the Company with or into another individual or corporation, partnership, trust, incorporated or
unincorporated association, joint venture, limited liability company, joint stock company, government (or an agency or subdivision thereof)
or other entity of any kind (a “Person”), (ii) the Company (and all of its subsidiaries, taken as a whole), directly or indirectly,
effects any sale, lease, license, assignment, transfer, conveyance or other disposition of all or substantially all of its assets in one
or a series of related transactions, (iii) any, direct or indirect, purchase offer, tender offer or exchange offer (whether by the Company
or another Person) is completed pursuant to which holders of the Company’s common stock are permitted to sell, tender or exchange
their shares for other securities, cash or property and has been accepted by the holders of fifty percent (
Voting Rights. The Series A Holders will have no voting rights, except as otherwise required by the Delaware General Corporation Law. Notwithstanding the foregoing, as long as any shares of Series A Preferred Stock are outstanding, the Company shall not, without the affirmative vote of the holders of a majority of the then outstanding shares of Series A Preferred Stock, voting as a separate class, (a) alter or change adversely the powers, preferences or rights given to the Series A Preferred Stock in the Series A Certificate of Designation, (b) increase the number of authorized shares of Series A Preferred Stock, (c) authorize or issue an additional class or series of capital stock that ranks senior to the Series A Preferred Stock with respect to the distribution of assets on liquidation or (d) enter into any agreement with respect to any of the foregoing.
F-27
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 14 – EQUITY (continued)
Series A Convertible Preferred Stock (continued)
Fractional Shares. No fractional shares or scrip representing fractional shares shall be issued upon the conversion of the Series A Preferred Stock. As to any fraction of a share of Company common stock which a Series A Holder would otherwise be entitled to upon such conversion, the Company will, at its election, either pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the Conversion Price or round up to the next whole share. Notwithstanding the foregoing, nothing shall prevent any Series A Holder from converting fractional shares of Series A Preferred Stock.
Series B Convertible Preferred Stock
As described in Note
20 - Amended and Restated Membership Interest Purchase Agreement, in conjunction with the transaction, on February 9, 2023, the Company
filed a Certificate of Designation of Preferences, Rights and Limitations of the Series B Preferred Stock (the “Series B Certificate
of Designation”), which became effective immediately with the Secretary of State of the State of Delaware. The Company designated
up to
The shares of Series B Preferred Stock have identical terms and include the terms as set forth below.
Dividends. The Series B Holders shall be entitled to receive, and the Company shall pay, dividends on shares of Series B Preferred Stock equal (on an as-if-converted-to-common-stock basis, disregarding for such purpose any conversion limitations set forth in the Series B Certificate of Designations) to and in the same form as dividends actually paid on shares of the Company’s common stock when, as and if such dividends are paid on shares of the common stock. No other dividends shall be paid on shares of Series B Preferred Stock. The Company will not pay any dividends on its common stock unless the Company simultaneously complies with the terms set forth in the Series B Certificate of Designation.
Rank. The Series B Preferred Stock will rank subordinate to the shares of the Company’s Series A Preferred Stock.
Liquidation. Upon
any Liquidation, the Series B Holders will be entitled to receive out of the assets available for distribution to stockholders, (i) after
and subject to the payment in full of all amounts required to be distributed to the holders of another class or series of stock of the
Company ranking on liquidation prior and in preference to the Series B Preferred Stock, including the Series A Preferred Stock, (ii) ratably
with any class or series of stock ranking on liquidation on parity with the Series B Preferred Stock and (iii) in preference and priority
to the holders of the shares of common stock, an amount equal to one hundred percent (
Conversion. Each
share of Series B Preferred Stock shall be convertible, at any time and from time to time from and after the later of (i) the date of
the stockholder approval and (ii) the one year anniversary of the Closing Date (the “Lock Up Period”), at the option of the
Series B Holder thereof, into that number of shares of common stock (subject to the limitations set forth in Series B Certificate of Designation
determined by dividing the Series B Stated Value of such share of Series B Preferred Stock by the conversion price of the Series B Preferred
Stock). Series B Holders may effect conversions by providing the Company with the form of conversion notice attached as Annex A to the
Series B Certificate of Designation. The Series B Preferred Stock will be convertible into shares of the Company’s common stock
at a conversion price per share equal to $
F-28
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 14 – EQUITY (continued)
Series B Convertible Preferred Stock (continued)
Conversion Price Adjustment:
Stock Dividends and Stock Splits. If the Company, at any time while the Series B Preferred Stock is outstanding: (i) pays a stock dividend or otherwise makes a distribution or distributions payable in shares of common stock on shares of common stock or any other common stock equivalents (which, for avoidance of doubt, shall not include any shares of common stock issued by the Company upon conversion of, or payment of a dividend on, the Series B Preferred Stock), (ii) subdivides outstanding shares of common stock into a larger number of shares, (iii) combines (including by way of a reverse stock split) outstanding shares of common stock into a smaller number of shares, or (iv) issues, in the event of a reclassification of shares of the common stock, any shares of capital stock of the Company, then the conversion price of the Series B Preferred Stock shall be multiplied by a fraction of which the numerator shall be the number of shares of common stock (excluding any treasury shares of the Company) outstanding immediately before such event, and of which the denominator shall be the number of shares of common stock outstanding immediately after such event. Any of the foregoing adjustments shall become effective immediately after the record date for the determination of stockholders entitled to receive such dividend or distribution and shall become effective immediately after the effective date in the case of a subdivision, combination or re-classification.
Fundamental Transaction.
If, at any time while the Series B Preferred Stock is outstanding, (i) the Company, directly or indirectly, in one or more related transactions
effects any merger or consolidation of the Company with or into another Person, (ii) the Company (and all of its subsidiaries, taken as
a whole), directly or indirectly, effects any sale, lease, license, assignment, transfer, conveyance or other disposition of all or substantially
all of its assets in one or a series of related transactions, (iii) any, direct or indirect, purchase offer, tender offer or exchange
offer (whether by the Company or another Person) is completed pursuant to which holders of the Company’s common stock are permitted
to sell, tender or exchange their shares for other securities, cash or property and has been accepted by the holders of fifty percent
(
Voting Rights. The Series B Holders will have no voting rights, except as otherwise required by the Delaware General Corporation Law. Notwithstanding the foregoing, in addition, as long as any shares of Series B Preferred Stock are outstanding, the Company shall not, without the affirmative vote of the holders of a majority of the then outstanding shares of the Series B Preferred Stock, voting as a separate class, (a) alter or change adversely the powers, preferences or rights given to the Series B Preferred Stock in the Series B Certificate of Designation, (b) increase the number of authorized shares of Series B Preferred Stock, (c) except with respect to the Series A Preferred Stock, authorize or issue an additional class or series of capital stock that ranks senior to the Series B Preferred Stock with respect to the distribution of assets on liquidation or (d) enter into any agreement with respect to any of the foregoing.
F-29
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 14 – EQUITY (continued)
Series B Convertible Preferred Stock (continued)
Fractional Shares. No fractional shares or scrip representing fractional shares shall be issued upon the conversion of the Series B Preferred Stock. As to any fraction of a share which a Series B Holder would otherwise be entitled to upon such conversion, the Company shall at its election, either pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the Conversion Price or round up to the next whole share. Notwithstanding the foregoing, nothing shall prevent any Series B Holder from converting fractional shares of Series B Preferred Stock.
Series A Convertible Preferred Stock Sold for Cash
During the year ended
December 31, 2022, the Company sold an aggregate of
The Company evaluated the features of the Series A Convertible Preferred Stock under ASC 480, and classified them as permanent equity because the Series A Convertible Preferred Stock is not mandatorily or contingently redeemable at the stockholder’s option and the liquidation preference that exists does not fall within the guidance of SEC Accounting Series Release No. 268 – Presentation in Financial Statements of “Redeemable Preferred Stocks” (“ASR 268”).
Common Shares Sold for Cash
On December 13, 2019, the Company entered into
an Open Market Sale AgreementSM (the “Sales Agreement”) with Jefferies LLC, as sales agent (“Jefferies”),
pursuant to which the Company may offer and sell, from time to time, through Jefferies, shares of its common stock. During the year ended
December 31, 2022, Jefferies sold an aggregate of
On August 5, 2022, the Company sold
On August 5, 2022, the Company sold
Common Shares Issued for Services
During the year ended December 31, 2022, the Company
issued a total of
During the year ended December 31, 2021, the Company
issued a total of
F-30
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 14 – EQUITY (continued)
Common Shares Issued for Settlement of Accrued Professional Fees
In June
2021, the Company issued
Common Shares Issued for Debt Conversion
On July
25, 2022, the Company and 2022 Convertible Note holder entered into a Conversion Agreement pursuant to which the investor converted its
Convertible Notes in the principal amount of $
Common Shares Issued Pursuant to Related Party Debt Settlement Agreement and Release
On July
25, 2022,
On
December 21, 2021, the Company and Mr. Lu entered into and closed a Debt Settlement Agreement and Release pursuant to which The Company
settled $
Options
The following table summarizes the shares of the Company’s common stock issuable upon exercise of options outstanding at December 31, 2022:
| Options Outstanding | Options Exercisable | |||||||||||||||||||||||
| Range of Exercise Price | Number Outstanding at December 31, 2022 | Weighted Average Remaining Contractual Life (Years) | Weighted Average Exercise Price | Number Exercisable at December 31, 2022 | Weighted Average Exercise Price | |||||||||||||||||||
| $ | $ | $ | ||||||||||||||||||||||
| $ | $ | $ | ||||||||||||||||||||||
F-31
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 14 – EQUITY (continued)
Options (continued)
Stock option activities for the years ended December 31, 2022 and 2021 were as follows:
| Number of Options | Weighted Average Exercise Price | |||||||
| Outstanding at January 1, 2021 | $ | |||||||
| Granted | ||||||||
| Expired | ( | ) | ( | ) | ||||
| Outstanding at December 31, 2021 | ||||||||
| Granted | ||||||||
| Expired | ( | ) | ( | ) | ||||
| Outstanding at December 31, 2022 | $ | |||||||
| Options exercisable at December 31, 2022 | $ | |||||||
| Options expected to vest | $ | |||||||
The aggregate intrinsic value of stock options
outstanding and stock options exercisable at December 31, 2022 was $
The fair values of options granted during the
year ended December 31, 2022 were estimated at the date of grant using the Black-Scholes option-pricing model with the following assumptions:
volatility of
The fair
values of options granted during the year ended December 31, 2021 were estimated at the date of grant using the Black-Scholes option-pricing
model with the following assumptions: volatility of
For the years ended December 31, 2022 and 2021,
stock-based compensation expense associated with stock options granted amounted to $
A summary of the status of the Company’s nonvested stock options granted as of December 31, 2022 and changes during the years ended December 31, 2022 and 2021 is presented below:
| Number of Options | Weighted Average Exercise Price | |||||||
| Nonvested at January 1, 2021 | $ | |||||||
| Granted | ||||||||
| Forfeited | ( | ) | ( | ) | ||||
| Vested | ( | ) | ( | ) | ||||
| Nonvested at December 31, 2021 | ||||||||
| Granted | ||||||||
| Vested | ( | ) | ( | ) | ||||
| Nonvested at December 31, 2022 | $ | |||||||
F-32
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 14 – EQUITY (continued)
Warrants
On March 28, 2022, the Company entered into Securities
Purchase Agreement with an accredited investor, which was amended on June 8, 2022, providing for the sale by the Company to the investor
of a Convertible Note in the amount of $
The fair
values of the warrants issued to the investor with this private placement were computed using the Black-Scholes option-pricing model with
the following assumptions: volatility of
There were no stock warrants issued, terminated/forfeited and exercised during the year ended December 31, 2021. Stock warrants activities during the year ended December 31, 2022 were as follows:
| Number of Warrants | Exercise Price | |||||||
| Outstanding at January 1, 2022 | $ | |||||||
| Issued | ||||||||
| Expired/exercised | ||||||||
| Outstanding and exercisable at December 31, 2022 | $ | |||||||
The following table summarizes the shares of the Company’s common stock issuable upon exercise of warrants outstanding at December 31, 2022:
| Warrants Outstanding | Warrants Exercisable | |||||||||||||||||
| Exercise Price | Number Outstanding at December 31, 2022 | Weighted Average Remaining Contractual Life (Years) | Number Exercisable at December 31, 2022 | Exercise Price | ||||||||||||||
| $ | $ | |||||||||||||||||
The aggregate intrinsic value of both stock warrants
outstanding and stock warrants exercisable at December 31, 2022 was $
NOTE 15 - STATUTORY RESERVE AND RESTRICTED NET ASSETS
The Company’s PRC subsidiary, Avalon Shanghai, is restricted in its ability to transfer a portion of its net asset to the Company. The payment of dividends by entities organized in China is subject to limitations, procedures and formalities. Regulations in the PRC currently permit payment of dividends only out of accumulated profits as determined in accordance with accounting standards and regulations in China.
The Company is required
to make appropriations to certain reserve funds, comprising the statutory surplus reserve and the discretionary surplus reserve, based
on after-tax net income determined in accordance with generally accepted accounting principles of the PRC (“PRC GAAP”). Appropriations
to the statutory surplus reserve are required to be at least
Relevant PRC laws and
regulations restrict the Company’s PRC subsidiary, Avalon Shanghai, from transferring a portion of its net assets, equivalent to
their statutory reserves and their share capital, to the Company’s shareholders in the form of loans, advances or cash dividends.
Only PRC entity’s accumulated profit may be distributed as dividend to the Company’s shareholders without the consent of a
third party. As of December 31, 2022 and 2021, total restricted net assets amounted to $
F-33
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 16 – NONCONTROLLING INTEREST
As of
December 31, 2022, Dr. Yu Zhou, former director and former co-chief executive officer of Genexosome, who owns
During the years ended December 31, 2022 and 2021, the Company did not allocate any net loss and foreign currency translation adjustment to the noncontrolling interest holder due to its inability to satisfy these deficits.
NOTE 17 – CONDENSED FINANCIAL INFORMATION OF THE PARENT COMPANY
Pursuant to the requirements
of Rule 12-04(a), 5-04(c) and 4-08(e)(3) of Regulation S-X, the condensed financial information of the parent company shall be filed when
the restricted net assets of consolidated subsidiary exceed
The Company performed
a test on the restricted net assets of consolidated subsidiary in accordance with such requirement and concluded that it was not applicable
to the Company as the restricted net assets of the Company’s PRC subsidiary did not exceed
NOTE 18 - CONCENTRATIONS
Customers
The following
table sets forth information as to each customer that accounted for
| Years Ended December 31, | ||||||||
| Customer | 2022 | 2021 | ||||||
| A (Hebei Daopei, a related party) | % | |||||||
| B | % | % | ||||||
| C | % | % | ||||||
| D | % | % | ||||||
| * |
Two customers,
of which, one is a related party and the other is a third party, whose outstanding receivable accounted for
Two customers,
of which, one is a related party and the other is a third party, whose outstanding receivable accounted for
Suppliers
No supplier
accounted for
F-34
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 19 – SEGMENT INFORMATION
For the year ended December 31, 2022 and 2021, the Company operated in two reportable business segments - (1) the real property operating segment, and (2) the medical related consulting services segment. The Company’s reportable segments are strategic business units that offer different services and products. They are managed separately based on the fundamental differences in their operations.
Due to the winding down of the medical related consulting services segment in 2022, the Company decided to cease all operations of this segment and no longer has any material revenues or expenses in this segment. As a result, commencing from the first quarter of 2023, the Company’s chief operating decision maker no longer reviews medical related consulting services operating results.
Information with respect to these reportable business segments for the years ended December 31, 2022 and 2021 was as follows:
| Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Revenues | ||||||||
| Real property operations | $ | $ | ||||||
| Medical related consulting services | ||||||||
| Total | ||||||||
| Costs and expenses | ||||||||
| Real property operations | ||||||||
| Medical related consulting services | ||||||||
| Total | ||||||||
| Gross profit | ||||||||
| Real property operations | ||||||||
| Medical related consulting services | ||||||||
| Total | ||||||||
| Other operating expenses | ||||||||
| Real property operations | ||||||||
| Medical related consulting services | ||||||||
| Corporate/Other | ||||||||
| Total | ||||||||
| Other (expense) income | ||||||||
| Interest expense | ||||||||
| Corporate/Other | ( | ) | ( | ) | ||||
| Total | ( | ) | ( | ) | ||||
| Other income (expense) | ||||||||
| Real property operations | ||||||||
| Medical related consulting services | ( | ) | ||||||
| Corporate/Other | ||||||||
| Total | ( | ) | ||||||
| Total other expense, net | ( | ) | ( | ) | ||||
| Net loss | ||||||||
| Real property operations | ||||||||
| Medical related consulting services | ||||||||
| Corporate/Other | ||||||||
| Total | $ | $ | ||||||
F-35
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 19 – SEGMENT INFORMATION (continued)
| Identifiable long-lived tangible assets at December 31, 2022 and 2021 | December 31, 2022 | December 31, 2021 | ||||||
| Real property operations | $ | $ | ||||||
| Medical related consulting services | ||||||||
| Corporate/Other | ||||||||
| Total | $ | $ | ||||||
| Identifiable long-lived tangible assets at December 31, 2022 and 2021 | December 31, 2022 | December 31, 2021 | ||||||
| United States | $ | $ | ||||||
| China | ||||||||
| Total | $ | $ | ||||||
NOTE 20 – COMMITMENTS AND CONTINCENGIES
Litigation
From time to time, the Company is subject to ordinary routine litigation incidental to its normal business operations. The Company is not currently a party to, and its property is not subject to, any material legal proceedings, except as set forth below.
On October
25, 2017, Genexosome entered into and closed a Stock Purchase Agreement with Beijing Genexosome and Yu Zhou, MD, PhD, the sole shareholder
of Beijing Genexosome, pursuant to which Genexosome acquired all of the issued and outstanding securities of Beijing Genexosome in consideration
of a cash payment in the amount of $
Operating Leases Commitment
The Company
is a party to leases for office space. These lease agreements will expire through February 2025. Rent expense under all operating leases
amounted to approximately $
Supplemental cash flow information related to leases for the years ended December 31, 2022 and 2021 is as follows:
| Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Cash paid for amounts included in the measurement of lease liabilities: | ||||||||
| Operating cash flows paid for operating lease | $ | $ | ||||||
| Right-of-use assets obtained in exchange for lease obligation: | ||||||||
| Operating lease | $ | $ | ||||||
F-36
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 20 – COMMITMENTS AND CONTINCENGIES (continued)
Operating Leases Commitment (continued)
The following table summarizes the lease term and discount rate for the Company’s operating lease as of December 31, 2022:
| Operating Lease | ||||
| Weighted average remaining lease term (in years) | ||||
| Weighted average discount rate | % | |||
The following table summarizes the maturity of lease liabilities under operating lease as of December 31, 2022:
| For the Year Ending December 31: | Operating Lease | |||
| 2023 | $ | |||
| 2024 and thereafter | ||||
| Total lease payments | ||||
| Amount of lease payments representing interest | ( | ) | ||
| Total present value of operating lease liabilities | $ | |||
| Current portion | $ | |||
| Long-term portion | ||||
| Total | $ | |||
Equity Investment Commitment
On May 29, 2018, Avalon
Shanghai entered into a Joint Venture Agreement with Jiangsu Unicorn Biological Technology Co., Ltd. (“Unicorn”), pursuant
to which a company named Epicon Biotech Co., Ltd. (“Epicon”) was formed on August 14, 2018. Epicon is owned
Joint Venture – Avactis Biosciences Inc.
On July 18, 2018, the
Company formed Avactis Biosciences Inc. (“Avactis”), a Nevada corporation, as a wholly owned subsidiary. On October 23, 2018,
Avactis and Arbele Limited (“Arbele”) agreed to the establishment of AVAR BioTherapeutics (China) Co. Ltd. (“AVAR”),
a Sino-foreign equity joint venture, pursuant to an Equity Joint Venture Agreement (the “AVAR Agreement”), which was to be
owned
On April 6, 2022, the
Company, Acactis, Arbele and Arbele Biotherapeutics Limited (“Arbele Biotherapeutics”), a wholly owned subsidiary of Arbele,
entered into an Amendment No. 1 to the Equity Joint Venture Agreement pursuant to which Arbele Biotherapeutics acquired
F-37
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 20 – COMMITMENTS AND CONTINCENGIES (continued)
Joint Venture – Avactis Biosciences Inc. (continued)
The Company is required
to contribute $
In addition, the Company is responsible for:
| ● | Contributing registered capital of RMB |
| ● | assist Avactis in setting up its business operations and obtaining all required permits and licenses from the Chinese government; |
| ● | assisting Avactis in recruiting, hiring and retaining personnel; |
| ● | providing Avactis with access to various hospital networks in China to assist in the testing and commercialization of the CAR-T/CAR-NK/TCR-T/universal cellular immunotherapy technology in China; |
| ● | assisting Avactis in managing the Good Manufacturing Practices (GMP) facility and clinic to be developed by Avactis; |
| ● | providing Avactis with advice pertaining to conducting clinicals in China; and |
| ● | Within 6 days of signing the AVAR Agreement, the Company is required to pay to Arbele Biotherapeutics $ |
Under AVAR Agreement, as amended, Arbele Biotherapeutics shall be responsible for the following:
| ● | Entering into a License Agreement with Avactis; and | |
| ● | Providing Avactis with research and development expertise pertaining to clinical laboratory medicine when hired by Avactis. |
As of both December 31, 2022 and 2021, the Company
paid the $
Line of Credit Agreement
On August 29, 2019, the Company entered into a
Line of Credit Agreement (the “Line of Credit Agreement”) providing the Company with a $
F-38
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 20 – COMMITMENTS AND CONTINCENGIES (continued)
Amended and Restated Membership Interest Purchase Agreement
On November 7, 2022,
Avalon Laboratory Services, Inc. (the “Buyer”), a wholly-owned subsidiary of Avalon GloboCare Corp. (the “Company”),
entered into a Membership Interest Purchase Agreement (the “MIPA”), by and among SCBC Holdings LLC (the “Seller”),
the Zoe Family Trust, and Bryan Cox and Sarah Cox as individuals (each an “Owner” and collectively, the “Owners”),
and Laboratory Services MSO, LLC (“Laboratory Services MSO”), pursuant to which, subject to the terms and conditions set forth
in the MIPA, the Buyer will acquire from the Seller, sixty percent (
On February 9, 2023 (the “Closing Date”), the Company entered into and closed an Amended and Restated Membership Interest Purchase Agreement (the “Amended MIPA”), by and among Avalon Laboratory Services, Inc., a wholly-owned subsidiary of the Company (the “Buyer”), SCBC Holdings LLC (the “Seller”), the Zoe Family Trust, Bryan Cox and Sarah Cox as individuals (each an “Owner” and collectively, the “Owners”), and Laboratory Services MSO, LLC (“Laboratory Services MSO”). The Amended MIPA amends and restates, in its entirety, that certain Membership Interest Purchase Agreement, dated November 7, 2023 (the “Original MIPA”).
Pursuant
to the terms and conditions set forth in the Amended MIPA, Buyer acquired from the Seller, forty percent (40%) of all the issued and outstanding
equity interests of Laboratory Services MSO (the “Purchased Interests”), free and clear of all liens (the “Transaction”).
The Amended MIPA contains customary representations and warranties and covenants. The Anniversary Payment and the Earnout Payments will be available to compensate the Buyer for certain losses it may incur pursuant the indemnification provisions set forth in the Amended MIPA.
In addition, at any time
during the period beginning on the Closing Date and ending on the date nine (9) months after the Closing Date, the Buyer, or its designated
affiliates under the Amended MIPA, may purchase from the Seller twenty percent (
F-39
AVALON GLOBOCARE CORP. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 21 – SUBSEQUENT EVENTS
The Company evaluated subsequent events and transactions that occurred after the balance sheet date up to the date that the financial statements were issued. Based upon this review, other than as described below, the Company did not identify any subsequent events that would have required adjustment or disclosure in the financial statements.
Reverse Stock Split
The Company effected a one-for-ten reverse stock split of its outstanding shares of common stock on January 5, 2023. The reverse split did not change the number of authorized shares of common stock or par value. All references in these consolidated financial statements to shares, share prices, exercise prices, and other per share information in all periods have been adjusted, on a retroactive basis, to reflect the reverse stock split.
Second Amended and Restated Limited Liability Company Agreement
In connection with the
Closing of the Transaction, Laboratory Services MSO entered into a Second Amended and Restated Limited Liability Company Agreement, dated
February 9, 2023 (the “Amended Operating Agreement”), by and among the Seller, the Zoe Family Trust, the Owners, and the members
named therein. The terms of the Amended Operating Agreement, include, but are not limited to: (i) establishing Laboratory Services MSO
as a multi-member entity as of the Closing Date of the Transaction; (ii) reaffirming the Buyer’s right to purchase an additional
twenty percent (
Common Shares Issued for Services
In March 2023, the Company issued a total of
Line of Credit
As disclosed elsewhere,
the Company entered into a Line of Credit Agreement (the “Line of Credit Agreement”) providing the Company with a $
F-40